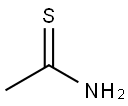
1H-Isoindole-1,3(2H)-dione, 2-(1-thioxoethyl)- synthesis
- Product Name:1H-Isoindole-1,3(2H)-dione, 2-(1-thioxoethyl)-
- CAS Number:2905-38-6
- Molecular formula:C10H7NO2S
- Molecular Weight:0
Yield:2905-38-6 19%
Reaction Conditions:
in dichloromethane at 0; for 40 h;
Steps:
81.1
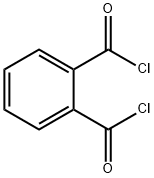
EXAMPLE 81; Preparation of N- ( (lS, 2R)-l- (3, 5-difluorobenzyl) -2- hydroxy-3- { [1- (3-isopropylphenyl) cyclohexyl] amino} propyl) ethanethioamide hydrochloride 46; Scheme 13 Step 1; Preparation of thioacetyl-N-phthalimide 45; Thioacetamide (1.9 g, 25 mmol) is suspended in 40 mL of CH2C12 and cooled in an ice bath under nitrogen. Phthaloyldichloride (3.6 mL, 25 mmol) is added slowly over 10 min via syringe while the mixture is stirred. The mixture becomes a clear orange solution transiently, eventually depositing a precipitate. After stirring for 40 h, the mixture is concentrated in vacuo (in the hood.). The oily coral solid is triturated with hexanes. Within minutes the hexanes mother liquor drops a precipitate, which is filtered off to afford 0.2 g of a light coral solid : 1H NMR (CDCl3) 8 7.99 (m, 2 H), 7. 86 (m, 2 H), 3.08 (s, 3 H). The residual solids remaining after trituration with hexanes are further triturated with ether and then with CH2Cl2. The combined mother liquors are concentrated to about 3 g of a red oily solid, which is chromatographed over silica gel, eluting with 10% to 20% ethyl acetate in heptane. The red fractions contained a product (concentrated to a coral solid, 0.77 g) with the same TLC retention (Rf = 0.32, 20% ethyl acetate in heptane) as the coral solid which had precipitated from hexanes. The total recovery is 0.97 g, 4.7 mmol, 19%.
References:
WO2005/95326,2005,A2 Location in patent:Page/Page column 200-201