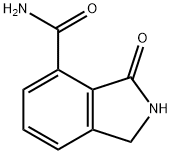
1H-Isoindole-4-carboxaMide, 2,3-dihydro-3-oxo- synthesis
- Product Name:1H-Isoindole-4-carboxaMide, 2,3-dihydro-3-oxo-
- CAS Number:935269-26-4
- Molecular formula:C9H8N2O2
- Molecular Weight:176.17
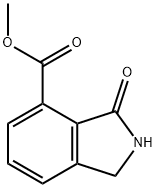
935269-25-3
36 suppliers
$188.00/100mg

935269-26-4
17 suppliers
$60.00/5mg
Yield:-
Reaction Conditions:
with ammonia in tetrahydrofuran;water at 20; for 0.5 h;
Steps:
16
Compound 16d was dissolved in THF and treated with concentrated ammonium hydroxide at room temperature for 30 min to give 3-oxo-2,3-dihydro-lH-isoindole-4- carboxylic acid amide 16e 1H NMR (300 MHz, DMSO-^6) 4.64 (s, 2H), 7.84 (t, J = 7.6 Hz, IH), 7.92 (d, J = 7 Hz, IH), 8.17 (d, J = 7.7 Hz, IH), 10.15 (broad s, IH); MS (ESI) m/z: 177 (MH+). )
References:
Location in patent:Page/Page column 68-69