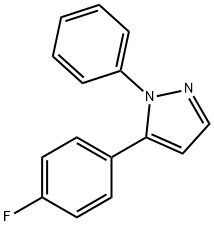
1H-Pyrazole, 5-(4-fluorophenyl)-1-phenyl- synthesis
- Product Name:1H-Pyrazole, 5-(4-fluorophenyl)-1-phenyl-
- CAS Number:439289-13-1
- Molecular formula:C15H11FN2
- Molecular Weight:238.26

75175-77-8
48 suppliers
$5.00/250mg
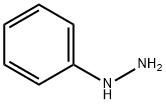
100-63-0
362 suppliers
$10.00/1g

439289-13-1
1 suppliers
inquiry
Yield:439289-13-1 83%
Reaction Conditions:
with C24H35N2O6(1+)*HO4S(1-) at 50; for 0.333333 h;Green chemistry;
Steps:
General procedure for the synthesis of pyrazole derivatives (3a-3p)
General procedure: In a 50 ml round-bottom flask, substituted enaminones (1 mmol), hydrazine hydrate (1.3 mmol) or phenyl hydrazine (1 mmol) and [crown ether MIm][HSO4] (20 mol%) were charged. The reaction mixture was stirred at 50 C under solvent-free conditions for 10-35 min. After completion of reaction (monitoredby TLC), the reaction mixture was extracted with ethyl acetate (2 9 10 ml). The extracted ethyl acetate was evaporated in a rotary evaporator and the desired product was isolated from the resultant mass by column chromatography (petether: ethyl acetate, 9.5: 0.5). Finally, the catalyst was recycled and used for thenext run.
References:
Patil, Dayanand;Chandam, Dattatraya;Mulik, Abhijeet;Jagdale, Suryabala;Patil, Prasad;Deshmukh, Madhukar [Research on Chemical Intermediates,2015,vol. 41,# 9,art. no. 1782,p. 6843 - 6858]