1H-Tetrazole, 1-(phenylmethyl)- synthesis
- Product Name:1H-Tetrazole, 1-(phenylmethyl)-
- CAS Number:6926-50-7
- Molecular formula:C8H8N4
- Molecular Weight:160.18
Yield:6926-50-7 96%
Reaction Conditions:
with Caswell No. 744A;1,1',1''?(1,3,5?triazine?2,4,6?triyl)tris(3?methyl?1H?imidazol?3?ium) iodide at 60; for 3 h;Sonication;
Steps:
General procedure for ultrasound-assisted preparation of 5-substituted1H-tetrazoles from aldehyde
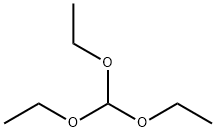
General procedure: To synthesis of 5-substituted 1H-tetrazoles from aldehyde precursor, a mixture of aldehyde (1.0 mmol), and hydroxyl amine hydrochloride (1.5 mmol) in TAIm[I](2.5 mL) was ultrasonicated at 60 °C for 10 min. Then, NaN3(1.5 mmol) was added to the mixture. The reaction progress was monitored by TLC. Upon the reaction completion, hot H2O(3.0 mL) and hot EtOAc (3.0 mL) was added to the mixture,and the product was further extracted three times to EtOAc. The resulting combined organic layers were dried over MgSO4.The desired 5-substituted 1H-tetrazole product was purified by recrystallization in EtOH. The ionic liquid that is now as TAIm[N3] form (see recycling and mechanism studies), was efficiently recycled from the aqueous phase by extraction to pure n-BuOH (3 × 6.0 mL) followed by removal of n-BuOH under reduced pressure [38]. For the preparation of 5-substituted 1H-tetrazoles from nitrile, aryl nitrile(1.0 mmol) was used as a precursor under ultrasonic at 60 °C. A same procedure was applied for ultrasonic-assisted preparation of 1-substituted 1H-1,2,3,4-tetrazoles by amine (1.0 mmol) and triethyl orthoformate (1.2 mmol).
References:
Jasim, Saade Abdalkareem;Tanjung, Faisal Amri;Sharma, Sandhir;Mahmoud, Mustafa Z.;Kadhim, Sally B.;Kazemnejadi, Milad [Research on Chemical Intermediates,2022,vol. 48,# 8,p. 3547 - 3566]
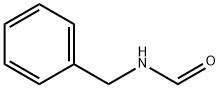
6343-54-0
103 suppliers
$7.00/1g

6926-50-7
7 suppliers
inquiry
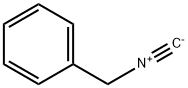
10340-91-7
126 suppliers
$81.00/1g

6926-50-7
7 suppliers
inquiry

100-39-0
434 suppliers
$10.00/10g

6926-50-7
7 suppliers
inquiry