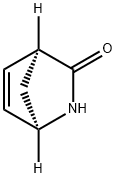
((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one synthesis
- Product Name:((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one
- CAS Number:79200-56-9
- Molecular formula:C6H7NO
- Molecular Weight:109.13
134003-04-6
103 suppliers
$82.00/100mg
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
226 suppliers
$10.00/1g
Yield:98 % ee
Reaction Conditions:
with (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate in N,N-dimethyl-formamide at 45; for 15 h;
Steps:
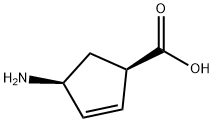
1.6; 2.6; 3.6 (6) (1R, 4S) 2-azabicyclo[2.2.1]hept-5-en-3-one
Dissolve 2.5 g of (1R, 4S) 4-aminocyclopent-2-ene-1-carboxylic acid in 10 mL of N, N-dimethylformamide, and add three7.4g of pyrrolidinylphosphonium bromide hexafluorophosphate, heated at 45°C to react for 15H. After the reaction, add water to dilute and extract with ethyl acetate. Take, wash the organic phase once with saturated ammonium chloride, wash once with saturated sodium bicarbonate, then dry the organic phase and spin dry to obtain the crude product. Ethyl acetate/n-hexane system is crystallized to obtain refined products with an ee value of 98%.
References:
CN111072547,2020,A Location in patent:Paragraph 0023; 0038-0040; 0041; 0056-0058; 0059; 0074-0076
![2-Piperidinone, 5-hydroxy-6-[(phenylmethoxy)methyl]-, (5S-trans)- (9CI)](/CAS/20210305/GIF/168773-36-2.gif)
168773-36-2
0 suppliers
inquiry
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
226 suppliers
$10.00/1g
![2-Piperidinone, 5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-6-[(phenylmethoxy)methyl]-, (5S-trans)- (9CI)](/CAS/20210305/GIF/168773-38-4.gif)
168773-38-4
0 suppliers
inquiry
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
226 suppliers
$10.00/1g
![2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/49805-30-3.gif)
49805-30-3
364 suppliers
$5.00/5g
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
226 suppliers
$10.00/1g
![(1S,4R)-2-Aza-bicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/130931-83-8.gif)
130931-83-8
166 suppliers
$8.00/100mg
![(1R,4S,6S)-6-((tert-Butyldimethylsilyl)oxy)-2-azabicyclo[2.2.1]heptan-3-one](/CAS/20211123/GIF/168773-48-6.gif)
168773-48-6
1 suppliers
inquiry
![((1R,4S)-2-Azabicyclo[2.2.1]hept-5-en-3-one](/CAS/GIF/79200-56-9.gif)
79200-56-9
226 suppliers
$10.00/1g