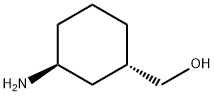
((1S,3S)-3-aminocyclohexyl)methanol synthesis
- Product Name:((1S,3S)-3-aminocyclohexyl)methanol
- CAS Number:1202411-97-9
- Molecular formula:C7H15NO
- Molecular Weight:129.2
![Carbamic acid, N-[(1S,3S)-3-(hydroxymethyl)cyclohexyl]-, phenylmethyl ester](/CAS/20210305/GIF/1202411-96-8.gif)
1202411-96-8
0 suppliers
inquiry

1202411-97-9
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol at 20; under 2585.81 Torr; for 4 h;
Steps:
83
Preparation 83
References:
WO2009/153720,2009,A1 Location in patent:Page/Page column 247

116724-13-1
5 suppliers
inquiry

1202411-97-9
2 suppliers
inquiry