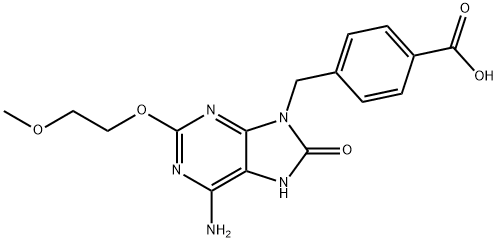
1V209 synthesis
- Product Name:1V209
- CAS Number:1062444-54-5
- Molecular formula:C16H17N5O5
- Molecular Weight:359.34
![Benzonitrile, 4-[[6-amino-8-methoxy-2-(2-methoxyethoxy)-9H-purin-9-yl]methyl]-](/CAS/20210111/GIF/927822-43-3.gif)
927822-43-3
0 suppliers
inquiry

1062444-54-5
26 suppliers
inquiry
Yield: 85%
Reaction Conditions:
Stage #1:4-((6-amino-8-methoxy-2-(2-methoxyethoxy)-9H-purin-9-yl)methyl)benzonitrile with water;sodium hydroxide in ethanol for 8 h;Reflux;
Stage #2: with hydrogenchloride in ethanol;water; pH=2
Steps:
1.d
(d) NaOH:EtOH 1:1, reflux; Synthesis of 4-((6-amino-2-(2-methoxyethoxy)-8-oxo-7H-purin-9(8H)-yl)methyl)benzoic acid (see FIG. 1, compound 5). 20 mL of a 1:1 ethanol:water mixture was added to 0.10 g (0.28 mmol) of 4-((6-amino-8-methoxy-2-(2-methoxyethoxy)-9H-purin-9 yl)methyl)benzonitrile (see FIG. 1, compound 1), and the combination refluxed for 8 hours. The reaction mixture was allowed to cool and acidified to pH 2 with conc. HCl. The aqueous solution was further extracted with DCM (3×20 mL), dried over MgSO4 and evaporated in vacuo to yield a mixture of 8-oxo-9-benzoic acid (compound 5), 8-methoxy-9-benzoic acid and 8-oxo-9-ethyl benzoate. Once dried, the products were dissolved in CH3CN (25 mL) and NaI (0.14 g, 0.96 mmol) was added (FIG. 1, reaction step (b)). To this solution was added 12 μL (0.96 mmol) of chlorotrimethylsilane, dropwise with stirring. The reaction mixture was heated at 40° C. for 4 hours then cooled, filtered and washed with water (20 mL) and then diethyl ether (20 mL) to obtain a white solid in 85% yield. Nuclear Magnetic Resonance (NMR) analysis was performed on the resultant product, with the following results, 1H NMR (400 MHz, DMSO-d6) δ (ppm): 10.33 (s, 1H), 7.89 (d, J=8 Hz, 2H), 7.37 (d, J=8 Hz, 2H), 6.65 (s, 2H), 4.92 (s, 2H), 4.24 (t, J=4 Hz, 2H), 3.56 (t, J=4 Hz, 2H), 3.25 (s, 3H). Retention time (Rt) on HPLC=14.3 min. ESI-MS (positive ion mode): calculated for C16H17N5O5 m/z [M+1] 360.34; found 360.24.
References:
Regents of the University of California, San Diego US2010/210598, 2010, A1 Location in patent:Page/Page column title page; 13