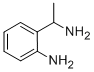
2-(1-AMINO-ETHYL)-PHENYLAMINE synthesis
- Product Name:2-(1-AMINO-ETHYL)-PHENYLAMINE
- CAS Number:39909-26-7
- Molecular formula:C8H12N2
- Molecular Weight:136.19
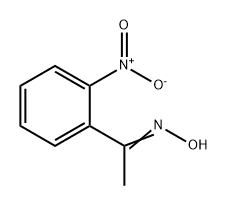
10342-62-8
0 suppliers
inquiry

39909-26-7
21 suppliers
inquiry
Yield:39909-26-7 73%
Reaction Conditions:
with hydrogenchloride;zinc in ethanol;water at 0 - 50; for 2 h;
Steps:
Method B
To a solution of 1-(2-nitrophenyl)ethan-1-one oxime (2.07 g, 11.5 mmol) in a mixtureof ethanol (45 mL), water (12 mL) and concentrated HCl (24 mL) stirred at 0 C zinc ductwas added in small portions over 30 min (9.03 g, 138.1 mmol) [43]. The reaction mixturewas stirred at 50 °C for 1.5 h (the reaction progress was monitored with TLC), then cooleddown to room temperature, and filtered. The filtrate was concentrated in vacuum, andthe residue was basied with aqueous ammonia (25%) to pH 9 and extracted with ethylacetate (3 50 mL). Combined organic extracts were dried with sodium sulphate andconcentrated in vacuum. The residual amber-colored oil was dissolved in diluted aqueousHCl (70 mL, pH of resulting solution c.a. 4±5), and washed with ethyl acetate (3 25 mL).The aqueous phase was basied with ammonia (25%) to pH 9 and extracted with ethylacetate (3 25 mL). Combined organic phases were washed with brine, dried with sodiumsulphate, and concentrated. 2-(1-Aminoethyl)aniline was obtained as pale-yellow oil,slowly solidifying upon standing. Grinding of this crystalline mass afforded pale-yellowpowder, mp 85-89 °C. Yield: 1.19 g (8.4 mmol, 73%). 1H NMR (400 MHz, DMSO-d6) δ 7.04(dd, J = 7.5, 1.6 Hz, 1H), 6.88 (td, J = 7.6, 1.6 Hz, 1H), 6.56 (dd, J = 7.9, 1.3 Hz, 1H), 6.48 (td,J = 7.4, 1.3 Hz, 1H), 4.03 (q, J = 6.6 Hz, 1H), 1.26 (d, J = 6.6 Hz, 3H).
References:
Aksenov, Alexander V.;Aksenov, Dmitrii A.;Aksenov, Nicolai A.;Grishin, Igor Yu.;Malyuga, Vladimir V.;Nobi, Mezvah A.;Rubin, Michael [Molecules,2021,vol. 26,# 14]
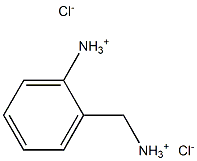
75191-79-6
4 suppliers
inquiry

39909-26-7
21 suppliers
inquiry
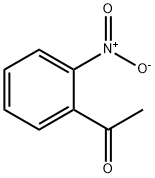
577-59-3
276 suppliers
$6.00/5g

39909-26-7
21 suppliers
inquiry
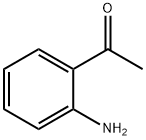
551-93-9
484 suppliers
$6.00/5g

39909-26-7
21 suppliers
inquiry
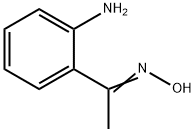
4964-49-2
6 suppliers
inquiry

39909-26-7
21 suppliers
inquiry