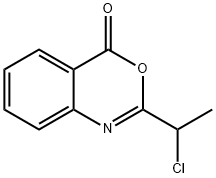
2-(1-CHLOROETHYL)QUINAZOLIN-4(3H)-ONE synthesis
- Product Name:2-(1-CHLOROETHYL)QUINAZOLIN-4(3H)-ONE
- CAS Number:137225-34-4
- Molecular formula:C10H9ClN2O
- Molecular Weight:208.64
Yield:137225-34-4 74%
Reaction Conditions:
at 80; for 1 h;
Steps:
2.2.1. General procedure for the synthesis of 2-benzopyrimidinones 2
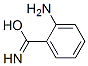
General procedure: A mixture of 2-aminobenzamide 1 (1 g, 4.9 mmol) and acyl chloride (1 mL, 5.8 mmol) was heated at 80 °C for 1 hour on steam bath.After the reaction was completed, the mixture was cooled to room temperature and neutralized with Na 2 CO 3 (10%) solution. The precipitate was collected and recrystallized from ethanol.
References:
Chortani, Sarra;Horchani, Mabrouk;Znati, Mansour;Issaoui, Noureddine;Jannet, Hichem Ben;Romdhane, Anis [Journal of Molecular Structure,2021,vol. 1230,art. no. 129920]
89441-13-4
0 suppliers
inquiry

137225-34-4
16 suppliers
inquiry
![2-[(2-Chloropropanoyl)amino]benzoic acid](/CAS/20140525/GIF/CB12689134.gif)
137225-33-3
6 suppliers
inquiry

137225-34-4
16 suppliers
inquiry