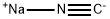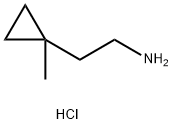
2-(1-methylcyclopropyl)ethan-1-amine hydrochloride synthesis
- Product Name:2-(1-methylcyclopropyl)ethan-1-amine hydrochloride
- CAS Number:1454690-72-2
- Molecular formula:C6H14ClN
- Molecular Weight:135.64
Yield:1454690-72-2 31%
Reaction Conditions:
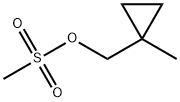
Stage #1: sodium cyanide;(1-methylcyclopropyl)methyl methanesulfonate in dimethyl sulfoxide at 20; for 4 h;
Stage #2: with dimethylsulfide borane complex in tetrahydrofuran at 20 - 65;
Stage #3: with hydrogenchloride
Steps:
126 Synthesis of 2-(1-methylcyclopropyl)ethanamine hydrochloride
Treat a solution of (l-methylcyclopropyl)methyl methanesulfonate (1.85 g, 11.27 mmol) in DMSO (20 mL) with sodium cyanide (1.104 g, 22.53 mmol) and stir at RT for 4 h. Add H20, extract with EtOAc (3x), wash the combined organics with brine, dry over Na2S04 and concentrate carefully to afford a colorless oil. Dissolve the oil in THF (15 mL), add borane dimethylsulfide complex (2.0M in THF, 8.45 mL, 16.90 mmol), heat at 65°C for 4 h, then cool to RT overnight. Concentrate the mixture to dryness, co- evaporate with EtOAc, triturate with ethyl ether, collect the solid via filtration and dry to afford the title compound (485 mg, 31%). 1H NMR (400 MHz, DMSO-d6): δ 7.90 (s, 2 H), 2.82 (m, 2 H), 1.48 (m, 2 H), 0.99 (s, 3 H), 0.31-0.22 (m, 4 H).
References:
WO2013/134298,2013,A1 Location in patent:Page/Page column 77