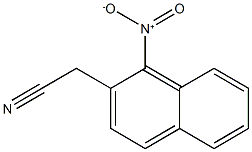
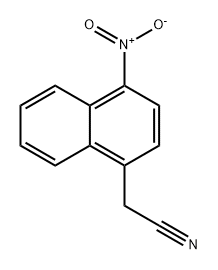
2-(1-nitronaphthalen-2-yl)acetonitrile synthesis
- Product Name:2-(1-nitronaphthalen-2-yl)acetonitrile
- CAS Number:89278-00-2
- Molecular formula:C12H8N2O2
- Molecular Weight:212.2041
Yield:89278-00-2 87%
Reaction Conditions:
with potassium tert-butylate in N,N-dimethyl-formamide at -24; for 2.5 h;
Steps:
Typical procedure for synthesis of nitriles:
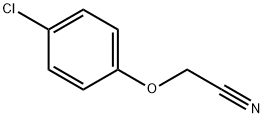
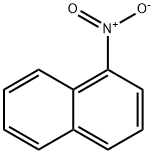
General procedure: 1 equivalent of the aromatic nitro compound and 1.2 equivalents of4-chlorophenoxyacetonitrile were dissolved in anhydrous DMF and slowly added toa solution of 2.3 equivalents of potassium tert-butoxidedissolved in DMF at -24 °C. The color of the mixture turned to deep purple, andit was stirred at -24 °C for further 2.5 h. Then the reaction mixture waspoured into 200 ml of ice cold 5 % HCl. A brown precipitate was formed. Themixture was extracted with ethyl acetate (EA) and the product purified by flashchromatography.
References:
Schlosser, Joachim;Johannes, Eugen;Zindler, Melanie;Lemmerhirt, Jan;Sommer, Benjamin;Schütt, Martin;Peifer, Christian [Tetrahedron Letters,2015,vol. 56,# 1,p. 89 - 94] Location in patent:supporting information
86-57-7
6 suppliers
$14.00/5g

107-14-2
274 suppliers
$15.00/25g

89278-00-2
5 suppliers
inquiry
72301-67-8
0 suppliers
inquiry

35120-10-6
67 suppliers
$21.50/5g

86-57-7
6 suppliers
$14.00/5g

89278-00-2
5 suppliers
inquiry

72301-67-8
0 suppliers
inquiry

86-57-7
6 suppliers
$14.00/5g

3598-14-9
83 suppliers
$28.28/5gm:

89278-00-2
5 suppliers
inquiry

72301-67-8
0 suppliers
inquiry