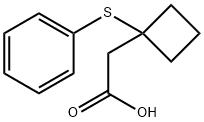
2-[1-(phenylsulfanyl)cyclobutyl]acetic acid synthesis
- Product Name:2-[1-(phenylsulfanyl)cyclobutyl]acetic acid
- CAS Number:934555-27-8
- Molecular formula:C12H14O2S
- Molecular Weight:222.3
Yield:-
Reaction Conditions:
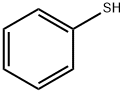
Stage #1: thiophenolwith potassium carbonate in tetrahydrofuran;water; for 1 h;Heating / reflux;
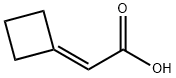
Stage #2: 2-cyclobutylideneacetic acid in tetrahydrofuran;water;N,N-dimethyl-formamide; for 72 h;
Steps:
81.I
Example 81 : (+/-) l-fS^-dihydro-spiro^H-l-benzothiopyran^.r-cyclobutani^-yD-S-( isoquinolin-5 -yPureaStep I: l-Phenylthiocyclobutane-l -acetic acidA solution of thiophenol (70 mmol) in THF (10 ml) was refluxed for Ih in the presence of of K2CO3 (70 mmol) as a base. A solution of Cyclobutylidene acetic acid (intermediate 1) (35 mmol) in DMF (1.0 ml) was added to the above reaction. The reaction was monitored by TLC for completion. After 3 days the reaction was cooled and filtered. It was then neutralized with EPO
References:
WO2007/42906,2007,A1 Location in patent:Page/Page column 77-78