2(1H)-Quinolinone, 8-chloro-4-hydroxy- synthesis
- Product Name:2(1H)-Quinolinone, 8-chloro-4-hydroxy-
- CAS Number:1900-41-0
- Molecular formula:C9H6ClNO2
- Molecular Weight:195.6
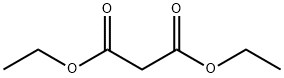
28272-93-7
14 suppliers
inquiry

1900-41-0
4 suppliers
inquiry
Yield:1900-41-0 87%
Reaction Conditions:
with polyphosphoric acid at 145; for 5 h;
Steps:
Synthesis of 8-chloro- 4-hydroxyquinoline -2-(1H)-one
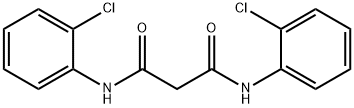
The N, N′-di-(2-chlorophenyl)malonamide (0.65g, 2.0mmol) and polyphosphoric acid (3.55g) were stirred in an oil bath for 5h at 145°C. Then the mixture was cooled, diluted with water and the resultant gum solidified by standing over night. The crude product was dissolved in 20mL of sodium hydroxide solution 0.1molL-1 and the residual was filtered off. The filtrate was acidified with concentrated hydrochloric acid and the resulting precipitate was recrystallized in a minimum amount of ethanol to afford 8-chloro-4-hydroxyquinoline-2-(1H)-one () as creamy crystals, Yield 87%, Mp: 302-303°C (reported 305°C [29]). FT-IR (KBr): ν(cm-1)=3470 (OH), 3100 (NH), 1635 (C=O); 1H NMR (400MHz, DMSO-d6); δ 11.86 (OH), 10.41 (NH), 7.81 (1H, d, J=8.0Hz), 7.67 (1H, d, J=7.6Hz), 7.18 (1H, dd, J=8.0, 7.6Hz), 5.83 (1H, s). 13C NMR (100MHz, DMSO-d6):163.94 (C=O), 162.30 (C-OH), 138.22 (C), 135.32 (C-Cl), 129.12 (CH), 126.20 (C-H), 125.17 (CH), 119.10 (C), 99.19 (CH). Anal. Calcd. for C9H6ClNO2: C, 55.26; H, 3.09; N, 7.16;. Found: C, 5.21; H, 3.06; N, 7.12.
References:
Yahyazadeh, Asieh;Yousefi, Hessamoddin [Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy,2014,vol. 117,p. 696 - 701]
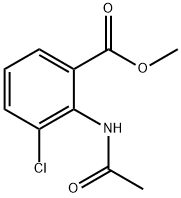
138825-97-5
0 suppliers
inquiry

1900-41-0
4 suppliers
inquiry
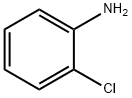
95-51-2
390 suppliers
$5.00/5G

1900-41-0
4 suppliers
inquiry