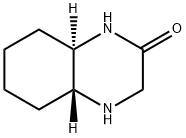
2(1H)-Quinoxalinone,octahydro-,(4aR-trans)-(9CI) synthesis
- Product Name:2(1H)-Quinoxalinone,octahydro-,(4aR-trans)-(9CI)
- CAS Number:32044-23-8
- Molecular formula:C8H14N2O
- Molecular Weight:154.21
Yield:32044-23-8 700 mg
Reaction Conditions:
with sodium hydrogencarbonate in tetrahydrofuran at 0 - 20; for 3 h;
Steps:
4.1 Step 1: Synthesis of (4aR,8aR)-Octahydroquinoxalin-2(1H)-one (198)
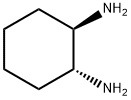
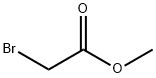
To a mixture of (1R,2R)-cyclohexane-1,2-diamine (197, 1 g, 8.75 mmol) and NaHCO3 (2.2 g, 26.2 mmol) in THF (40 mL) at 0 °C was added a solution of methyl 2-bromoacetate(1.34 g, 8.75 mmol) in THF (20 mL) in dropwise. After stirring at 0 °C for 2 h, the reaction mixture was warmed to room temperature and stirred for an additional 1 h. The reaction mixture was then quenched with water (40 mL) and extracted with EtOAc (50 mL x 2). The combined organic phases were dried over MgSO4, filtered and concentrated in vacuo. The resulting residue was purified by column chromatography to provide (4aR,8aR) -octahydroquinoxalin10 2(1H)-one (198, 700 mg, 4.54 mmol).
References:
WO2018/5860,2018,A1 Location in patent:Page/Page column 172; 175