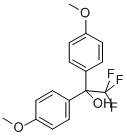
2,2,2-TRIFLUORO-1,1-BIS(4-METHOXYPHENYL)ETHANOL synthesis
- Product Name:2,2,2-TRIFLUORO-1,1-BIS(4-METHOXYPHENYL)ETHANOL
- CAS Number:379-21-5
- Molecular formula:C16H15F3O3
- Molecular Weight:312.28
Yield:379-21-5 99%
Reaction Conditions:
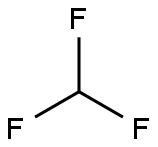
Stage #1: trifluoromethan;bis(p-methoxyphenyl)methanonewith tris(trimethylsilyl)amine;phosphazene base-P4-tert-butyl in tetrahydrofuran at 20; for 14 h;Schlenk technique;
Stage #2: with tetrabutyl ammonium fluoride in tetrahydrofuran at 20; for 1 h;Schlenk technique;
Steps:
4 Example 1 Preparation of 2,2,2-trifluoro-1,1-diphenylethanol (3)
A solution of benzophenone (36.4 mg, 0.20 mmol), tris (trimethylsilyl) amine (70.1 mg, 0.30 mmol, 1.5 equiv.) In THF (0.25 ml) was added to a Schlenk tube equipped with a stirrer at room temperature And P4-tBu base(50.0 μL, 0.040 mmol, 0.20 equiv.) Were charged, then trifluoromethane was bubbled through for 1 minute, and the reaction was carried out at the same temperature for 6 hours. After completion of the reaction, a saturated ammonium chloride aqueous solution was added. The mixture was extracted with methylene chloride, and the collected organic phases were washed with saturated brine and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and 2 mL of tetrahydrofuran and tetrabutylammonium fluoride (62.6 mg, 0.24 mmol, 1.2 equiv.) (Hereinafter abbreviated as TBAF) were added and stirred at room temperature for 1 hour . Thereafter, the solvent was distilled off under reduced pressure, and the obtained crude product was purified by silica gel column chromatographyTo obtain 2,2,2-trifluoro-1,1-diphenylethanol (3) as a target product in a yield of 84%.
References:
JP2016/204276,2016,A Location in patent:Paragraph 0029-0031; 0033-0035
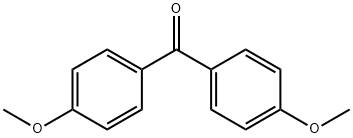
90-96-0
364 suppliers
$22.00/25g
1400691-55-5
7 suppliers
$55.39/1g

379-21-5
17 suppliers
$603.00/5g

90-96-0
364 suppliers
$22.00/25g

379-21-5
17 suppliers
$603.00/5g