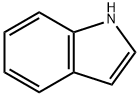
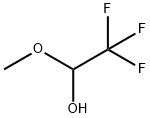
2,2,2-TRIFLUORO-1-(1H-INDOL-3-YL)-ETHANOL synthesis
- Product Name:2,2,2-TRIFLUORO-1-(1H-INDOL-3-YL)-ETHANOL
- CAS Number:116757-85-8
- Molecular formula:C10H8F3NO
- Molecular Weight:215.17
Yield:116757-85-8 100 %Chromat.
Reaction Conditions:
with triethylamine in hexane at 80;Microwave irradiation;Solvent;
Steps:
Typical reaction conditions
General procedure: Indole (0.05 g, 1 eq.), trifluoroacetaldehyde methyl hemiacetal (315 μL, 7 eq.), triethylamine (6.4 μL, 10 mol%), and the solvent (0.5 mL) were mixed together in a microwave vial (10 mL). This solution was irradiated in a closed vessel mode using a CEM Discover microwave reactor at 80 °C. Aliquots were withdrawn for analysis at desired times. The completion of the reaction was monitored by TLC and GC-MS. Products were purified by flash chromatography.
References:
Peerannawar, Swarada;Sood, Abha;Brown, Alicia;Sch?fer, Christian;Alonzo, Judith;Sutton, Steven;Christianson, Matthew;Stocking, Raven;Naclerio, Nicole;T?r?k, Béla;Landge, Shainaz M. [Structural Chemistry,2019,vol. 30,# 5,p. 1941 - 1956]