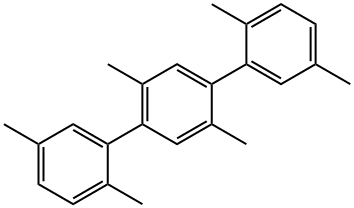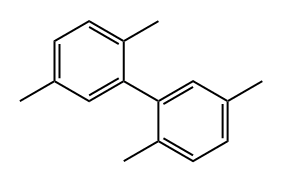
2,2',5,5'-TETRAMETHYLBIPHENYL synthesis
- Product Name:2,2',5,5'-TETRAMETHYLBIPHENYL
- CAS Number:3075-84-1
- Molecular formula:C16H18
- Molecular Weight:210.31
553-94-6
202 suppliers
$10.00/5g
30897-86-0
51 suppliers
$130.00/10g

3075-84-1
35 suppliers
$60.20/1g
Yield:3075-84-1 59%
Reaction Conditions:
with triphenylphosphine;nickel dibromide in o-xylene at 50 - 52;Inert atmosphere;
Steps:
3 Example 3 - Reaction of 2-Bromomagnesium-1 ,4-dimethylbenzene with 2-Bromo-1 ,4-dimethylbenzene in the Presence of 10/1 TPP / NiBr2
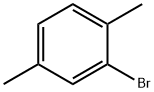
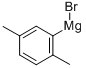
This example illustrates that the catalyst of this disclosure is also an effective coupling catalyst for preparing symmetrical sterically hindered bi- aryl compounds by mono-arylation reactions. The Grignard prep was carried out in a 1 -liter three-necked flask equipped with a chilled glycol reflux condenser, nitrogen atmosphere, an efficient Teflon-coated magnetic stirrer, thermowell, magnetic stirrer, heating mantle and a 250 ml pressure equalizing addition funnel. Magnesium metal shavings (7 grams / 0.288 mole), a crystal of iodine, and 150 ml of dry THF solvent were added to the flask. The addition funnel was charged with 2-bromo- 1 ,4-dimethylbenzene (46.3 grams / 0.25 mole) and dissolved in 100 ml of dry THF. The Grignard reaction was initiated by bringing the reaction flask temperature to 50 degrees Celsius and adding 5 ml of the bromo-xylene solution. After the initiation, the reaction temperature was kept at 50 - 55 degrees Celsius for the duration of the Grignard formation. The bromo-xylene was added dropwise over approximately 2 hours. The mixture was kept at 50 degrees Celsius for an additional hour to complete the reaction. The final solution was a light brown color. After cooling, the Grignard solution was transferred by cannula under nitrogen into a 500 ml pressure equalizing addition funnel. Approximately 15% of the Grignard solution was lost to a leak in the stopcock. The coupling reaction flask was a 1 liter three-necked flask equipped in the same manner as the Grignard flask. The 500 ml pressure equalizing addition funnel was also attached to one of the necks. Nickel bromide (0.55 grams / 2.5 mmole), triphenylphosphine (6.55 grams / 25 mmole), 2-bomo-1 ,4-dimethylbenzene (46.3 grams / 0.25 mole) and reagent grade o-xylene (50 ml / 44.3 grams) solvent were added. The catalyst was formed by heating the mixture to reflux and holding the temperature there for 30 minutes. After cooling to 50 degrees Celsius, 150 ml of dry THF solvent was added. The temperature in the flask was kept at 50 - 52 degrees Celsius for the rest of the coupling reaction. The Grignard solution was added dropwize over 3.5 hours. The green color turned to the characteristic red-brown color as the Grignard reagent was added. The mixture was stirred an additional two hours at 50 degrees to complete the reaction. After cooling to ambient temperature, the crude mixture was treated with a mixture of 1 15 ml of deionized water and 35 ml of concentrated hydrochloric acid and 3 ml of 50% aqueous hydrogen peroxide. The two phase mixture was separated in a separatory funnel. The organic layer was washed with two 100 ml portions of 2% aqueous NaCI. This material was analyzed by gas chromatography. The analysis indicated a conversion of 86% of the bromoxylene. The THF and most of the xylene solvent was removed by stripping with nitrogen at ambient temperature over night to form waxy low- melting point solid. The solid was dissolved in 120 ml of mixed hexane isomers and passed through a 1 " x 3" diameter bed of Brockmann I alumina. The bed was washed with 2 150 ml portions of a 2/1 v/v mixture of isomeric hexane / toluene. Nitrogen stripping was used as above to remove the large majority of hexane and toluene leaving 46.6 grams of a hard waxy solid. This material was distilled in a 2 cm diameter x 20 cm vacuum jacketed Vigreux column under vacuum. The desired product distilled overhead at a top take off temperature of 142 - 145 degrees Celsius at 9.6 mm mercury pressure (1 .28 kPa). The net weight of the hard waxy solid was 31 .2 grams or 59 % of theoretical yield. The purity of the fraction was 99.2%. The identity of the product was confirmed by GC-MS as being 2,2',5,5'-tetramethyl-1 ,1 '-biphenyl. The proton NMR spectrum in chemical shift relative to tetramethylsilane: 2 H (D) 7.12 ppm aromatic C-H; 2 H (D) 7.05 ppm aromatic C-H; 2 H (S) 6.92 ppm aromatic C-H; 6 H (S) 2.32 ppm 5,5' benzylic CH3; 6 H (S) 2.01 ppm 2,2' benzylic CH3.
References:
EASTMAN CHEMICAL COMPANY;DEVON, Thomas, James WO2018/44824, 2018, A1 Location in patent:Paragraph 0044; 0045; 0046; 0047
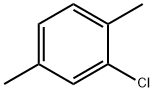
95-72-7
154 suppliers
$40.00/25g

3075-84-1
35 suppliers
$60.20/1g

553-94-6
202 suppliers
$10.00/5g

3075-84-1
35 suppliers
$60.20/1g
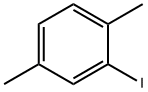
1122-42-5
74 suppliers
$10.00/1g

3075-84-1
35 suppliers
$60.20/1g