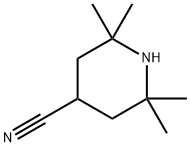
2,2,6,6-Tetramethyl-4-piperidinecarbonitrile synthesis
- Product Name:2,2,6,6-Tetramethyl-4-piperidinecarbonitrile
- CAS Number:67845-90-3
- Molecular formula:C10H18N2
- Molecular Weight:166.26
Yield:67845-90-3 90%
Reaction Conditions:
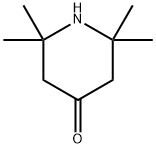
Stage #1: 2,2,6,6-Tetramethyl-4-piperidonewith potassium tert-butylate in 1,2-dimethoxyethane;tert-butyl alcohol; for 1 h;Inert atmosphere;
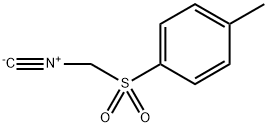
Stage #2: [(p-methylphenyl)sulfonylmethyl]isonitrile in 1,2-dimethoxyethane;tert-butyl alcohol at 20;Inert atmosphere;Reflux;
Steps:
Compound 2.
A solution of KOt-Bu (5190 mg, 46.3 mmol, 10 eq.) in 30 mL of t-BuOH was added to a solution of 2,2,6,6-Tetramethylpiperid-4-one (S.I.-2, 717 mg, 4.63 mmol) in 5 mL of DME and this solution was stirred for 1 h. Then a solution of TOSMIC (1806 mg, 9.25 mmol, 2S.I.-14 eq) in 5 mL of DME was added in one portion. The reaction was stirred at reflux for 2 h, after which time it was cooled to room temperature and allowed to stir for 14 h. The resulting solution was poured into 100 mL of ice water and extracted 3x with 50 mL of Et2O. The combined organic layers were washed with brine, dried over Na2SO4, filtered and the solvent was removed in vacuo to afford the intermediate nitrile 2,2,6,6-tetramethyl-4-cyanopiperidine in greater than 90% yield. This product was used without further purification. A 25 mL over-dried round bottom flask was charged with LiBH4 (60 mg, 2.77 mmol, 2 eq.) and suspended in 3 mL of THF. To this solution TMSCl (0.70 mL, 5.53 mmol, 4 eq.) was added added drop-wise over 5 min., during which the solution became cloudy. After stirring for 5 min, 2,2,6,6-tetramethyl-4-cyanopiperidine from above (230 mg, 1.38 mmol), dissolved in 1 mL of THF was added and the reaction was stirred at room temperature for 14 h. The reaction was then quenched by the addition of 10 mL of MeOH over 30 min., followed by removal of volatile products and solvent in vacuo. The resulting solid was suspended in 20 mL of 2 N KOH followed by extraction 3x with 20 mL of CH2Cl2, and the combined organic fractions were dried over Na2SO4, filtered, and the solvent was removed in vacuo, to obtain 216 mg of S.I.-3 as a waxy solid in greater than 90% yield and this product was used without further purification. Amine S.I.-3 (200 mg, 1.17 mmol) and ester S.I.-1 (288 mg, 1.17 mmol, 1 eq.) were coupled using the microwave procedure described in the synthesis of 1 to afford 229 mg of 2 (53 %) as an off-white solid.
References:
Lalonde, Judith M.;Elban, Mark A.;Courter, Joel R.;Sugawara, Akihiro;Soeta, Takahiro;Madani, Navid;Princiotto, Amy M.;Kwon, Young Do;Kwong, Peter D.;Sch?n, Arne;Freire, Ernesto;Sodroski, Joseph;Smith III, Amos B. [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 1,p. 91 - 101] Location in patent:supporting information; experimental part