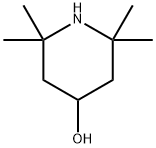
2,2,6,6-Tetramethyl-4-piperidinol synthesis
- Product Name:2,2,6,6-Tetramethyl-4-piperidinol
- CAS Number:2403-88-5
- Molecular formula:C9H19NO
- Molecular Weight:157.25
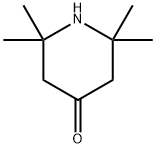
826-36-8
310 suppliers
$6.00/5g

2403-88-5
348 suppliers
$5.00/10g
Yield:2403-88-5 93%
Reaction Conditions:
with sodium tetrahydroborate in ethanol at 0 - 20; for 2 h;
Steps:
(2,2,6,6)-tetramethylpiperdin-4-ol
In a 500mL round bottom flask, 2,2,6,6-tetramethylpiperidin-4-one (15 g, 96.8 mmol) was diluted with 200 mL of absolute EtOH. The mixture was cooled to 0 °C and NaBH4 (1.5 equivalents, 5.5 g, 145 mmol) was carefully added portion wise (strong gas evolution). Reaction was allowed to reach room temperature under stirring for 2 hours and then cooled to 0 °C. Deionized water was slowly added (150 mL, white boron salt formation could be observed) and the mixture was warmed to room temperature overnight. After one extraction with AcOEt and concentration under vacuum the desired compound 1, was obtained as an orange solid (14.2 g, 93 %) and could be used without further purification. 1H NMR 400 MHz CDCl3 : δ 4.06 (m, 1H), 1.95 (dd, J=4.14 ; 12.47Hz, 2H), 1.33 (s, 1H), 1.24 (s, 6H), 1.11 (s, 6H), 1.0 (t, J=11.6Hz, 2H) ; 13C NMR 101 MHz CDCl3 : δ 64.5, 51.66, 48.15, 34.91, 28.70.
References:
Membrat, Romain;Vasseur, Alexandre;Giordano, Laurent;Martinez, Alexandre;Nuel, Didier [Tetrahedron Letters,2019,vol. 60,# 3,p. 240 - 243] Location in patent:supporting information

826-36-8
310 suppliers
$6.00/5g
36768-62-4
283 suppliers
$7.00/10g

2403-88-5
348 suppliers
$5.00/10g
67-64-1
0 suppliers
$17.30/10ml

2403-88-5
348 suppliers
$5.00/10g

2403-89-6
233 suppliers
$7.00/5g

2403-88-5
348 suppliers
$5.00/10g

826-36-8
310 suppliers
$6.00/5g

109-73-9
467 suppliers
$9.00/1g

2403-88-5
348 suppliers
$5.00/10g
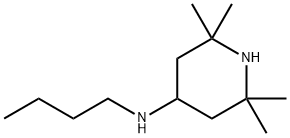
36177-92-1
51 suppliers
inquiry