
2-(2-AMINO-1,3-THIAZOL-4-YL)-4-CHLOROPHENOL synthesis
- Product Name:2-(2-AMINO-1,3-THIAZOL-4-YL)-4-CHLOROPHENOL
- CAS Number:175655-05-7
- Molecular formula:C9H7ClN2OS
- Molecular Weight:226.68
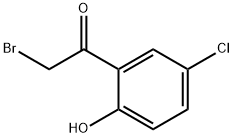
52727-99-8
30 suppliers
$38.00/1g

17356-08-0
3 suppliers
inquiry

175655-05-7
12 suppliers
$220.00/500mg
Yield:175655-05-7 64.5%
Reaction Conditions:
in ethanol; for 2 h;Heating / reflux;
Steps:
318.2
A mixture of 2-bromo-1-(5-chloro-2-hydroxyphenyl)ethanone(156.9mg, 0.63mmol), thiourea(47.9mg, 0.63mmol) and ethanol(3mL) was refluxed for 2 hours. After the reaction mixture was cooled to room temperature, it was poured into saturated sodium hydrogen carbonate solution and extracted with ethyl acetate. After the ethyl acetate layer was washed with brine and dried over anhydrous sodium sulfate, the residue obtained by evaporation of the solvent under reduced pressure was purified by column chromatography on silica gel(n-hexane:ethyl acetate=2:1) to give the title compound(98.6mg, 64.5%) as a light yellowish white powder.1H-NMR(DMSO-d6): δ 6.85(1H, d, J=8.7Hz), 7.14(1H, dd, J=8.7, 3.0Hz), 7.25(1H, s), 7.48(2H, s), 7.79(1H, d, J=3.0Hz), 11.95(1H, s).
References:
EP1510207,2005,A1 Location in patent:Page/Page column 166 EP1510210,2005,A1 Location in patent:Page/Page column 166
1450-74-4
256 suppliers
$8.00/5g

175655-05-7
12 suppliers
$220.00/500mg