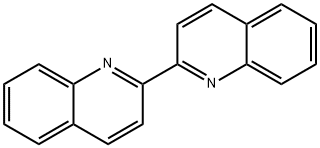
2,2'-Biquinoline synthesis
- Product Name:2,2'-Biquinoline
- CAS Number:119-91-5
- Molecular formula:C18H12N2
- Molecular Weight:256.3

612-62-4
256 suppliers
$35.00/5g

119-91-5
264 suppliers
$11.00/1g
Yield:119-91-5 91%
Reaction Conditions:
with bis(bipyridine)nickel(II) bromide;ethylene dibromide;sodium iodide in N,N-dimethyl-formamide at 20; for 2 h;Electrochemical reaction;Inert atmosphere;
Steps:
General procedure: DMF (40 mL), NaI (375 mg, 2.5 mmol), and 1,2-dibromoethane (100μL, 1.16 mmol) were added to an undivided electrochemical cell, fitted with an iron/nickel (64/36) anode, and surrounded by a nickel foam as the cathode (surface: 40 cm2, porosity: 500 μm, Goodfellow).The mixture was electrolyzed under argon at a constant current intensity of 0.2 A at r.t. for 15 min. The current was then stopped, then NiBr2bpy (187 mg, 0.5 mmol) and aryl or heteroaryl halide (5 mmol),were sequentially added. The solution was electrolyzed at 0.2 A untilthe starting aryl or heteroaryl halide had been totally consumed (2-5h). Sat. aq EDTA-Na2 solution (50 mL) was added, and the resultingsolution was extracted either with EtOAc (for aryl halides) or withCH2Cl2 (for heteroaryl halides) (3 × 50 mL). The combined organic layerswere washed with brine (50 mL), dried (MgSO4), filtered, and concentratedunder vacuum. The crude product was purified by flashchromatography (silica gel, 70-200 μm).
References:
Rahil, Rima;Sengmany, Stéphane;Le Gall, Erwan;Léonel, Eric [Synthesis,2018,vol. 50,# 1,art. no. SS-2017-N0393-OP,p. 146 - 154]
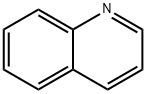
91-22-5
391 suppliers
$10.00/25g

119-91-5
264 suppliers
$11.00/1g

6907-48-8
0 suppliers
inquiry

119-91-5
264 suppliers
$11.00/1g
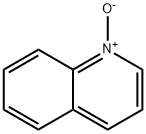
1613-37-2
4 suppliers
$53.00/5G

119-91-5
264 suppliers
$11.00/1g
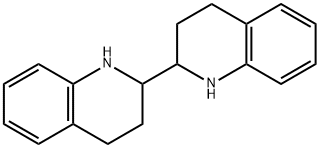
63469-73-8
0 suppliers
inquiry

119-91-5
264 suppliers
$11.00/1g