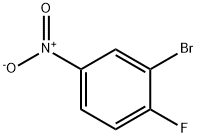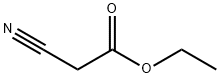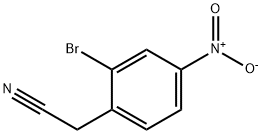
2-(2-bromo-4-nitrophenyl)acetonitrile synthesis
- Product Name:2-(2-bromo-4-nitrophenyl)acetonitrile
- CAS Number:543683-48-3
- Molecular formula:C8H5BrN2O2
- Molecular Weight:241.04
Yield:-
Reaction Conditions:
Stage #1: ethyl 2-cyanoacetatewith sodium hydride in 1,4-dioxane at 0; for 0.166667 h;
Stage #2: 2-bromo-1-fluoro-4-nitrobenzene in 1,4-dioxane at 20; for 16 h;
Steps:
111.A
A suspension of NaH (60% dispersion in mineral oil; 4.18 g; 110 mmol) in dioxane (40 mL) was chilled at 00C. A solution of ethyl-cyanoacetate (11.6 mL; 0.11 mmol) in dioxane (10 mL) was added by means of a dropping funnel under inert atmosphere over 30 min. After the reaction was stirred at 0°C for additional 10 min, 2-bromo-l- fluoro-4-nitro-benzene (8.00 g; 36.3 mmol) was added portionwise and the resulting dark solution was allowed to warm to room temperature and stirred for 16 hours. The reaction was carefully quenched with IN HCl and the solvent was removed under vacuum. The residue was taken up with EtOAc and washed twice with water. The organic phase was dried over Na2SO4, filtered and evaporated to dryness under vacuum. The resulting crude compound was dissolved in dioxane (30 mL) and then 37% HCl (10 mL) was added. The resulting yellow solution was refluxed for one day, then concentrated under reduced pressure. The resulting residue was taken up with ethyl ether, washed twice with 10% K2CO3 and extracted three times with diethyl ether. The ethereal phase was dried over Na2SO4, filtered and concentrated under reduced pressure. The crude compound was used in the next step without any further purification. LCMS (RT): 1.37 min (Method D).
References:
WO2008/117175,2008,A2 Location in patent:Page/Page column 143
555-21-5
42 suppliers
$21.00/25g

543683-48-3
23 suppliers
inquiry