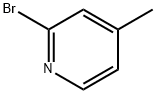
2-(2-BROMOPYRIDIN-4-YL)ACETONITRILE synthesis
- Product Name:2-(2-BROMOPYRIDIN-4-YL)ACETONITRILE
- CAS Number:312325-74-9
- Molecular formula:C7H5BrN2
- Molecular Weight:197.03
Yield:312325-74-9 62%
Reaction Conditions:
Stage #1: 2-Bromo-4-picoline;tert-Butoxybis(dimethylamino)methane at 110; for 15 h;
Stage #2: with hydroxylamine-O-sulfonic acid in water at 20; for 1 h;
Steps:
186.a
Example 186 4-[4-(2-Bromopyridyl)]-3-(4-fluorophenyl)-5-(phenylacetylamino)isoxazole a) 4-(2-Bromopyridyl)acetonitrile; A mixture of 1.5 g of 2-bromo-4-methylpyridine and 3.0 g of t-butoxybisdimethylaminomethane was stirred at 110°C for 15 hours. The reaction solution was cooled, and from which t-butoxybisdimethylaminomethane was distilled off under reduced pressure to provide dark brown oily residue. To this residue 20 mL of water and 2.5 g of hydroxylamine-O-sulfonic acid were added and stirred at room temperature for an hour. The crystals precipitated in the reaction solution were recovered by filtration, washed with water, and dried to provide 1.06 g (yield: 62%) of the title compound as brown crystals. 1H-NMR(CDCl3)δ:8.40(d,J=5.0Hz,1H), 7.51(dd,J=0.8Hz,1.5Hz,1H),7.27-7.24(m,1H),3.75(s,2H) Mass,m/e:196(M+),117(base)
References:
EP2036905,2009,A1 Location in patent:Page/Page column 45-46
118289-16-0
189 suppliers
$8.00/1g

312325-74-9
25 suppliers
inquiry