2-[(2-chlorophenyl)methylideneamino]guanidine synthesis
- Product Name:2-[(2-chlorophenyl)methylideneamino]guanidine
- CAS Number:13098-73-2
- Molecular formula:C8H9ClN4
- Molecular Weight:196.64

89-98-5
643 suppliers
$16.00/25g
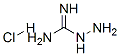
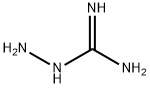
1937-19-5
229 suppliers
$5.00/100mg
![2-[(2-chlorophenyl)methylideneamino]guanidine](/CAS/20180702/GIF/13098-73-2.gif)
13098-73-2
22 suppliers
$45.00/10mg
Yield:13098-73-2 79.6%
Reaction Conditions:
with anhydrous Sodium acetate in ethanol at 25 - 80; for 6 h;
Steps:
Compound 1 : 2-(2-chlorobenzylidene)hydrazinecarboximidamide
General procedure: General procedure A: To a solution of benzaldehyde (1 eq.) in ethanol (300ml) was sequentially added Aminoguanidine hydrochloride (1 eq.) and sodium acetate (1 eq.) at 25°C. The resulting reaction mixture was heated at 80°C for next ~6 hours. Reaction completion was monitored on TLC using dichloromethane/methanol (8/2) as mobile phase. After completion of reaction, the reaction mixture was allowed to cool down to 25°C and dumped in the saturated solution of NaHC03 (700ml). The resulting precipitate were filtered off under vacuum and washed with water (100ml). The resulting solid material was titurated with diethylether (2 x 25 ml) and dried under vacumm to provide the desired substituted aminoguanidine derivative. The following compounds were prepared according general procedure A: Prepared following general procedure A from 2-chlorobenzaldehyde (10 g) to give 1 1 .1 g of desired compound (yield: 79.6%). 1 H-NMR (DMSO-d6): δ (ppm) 5.66 (s, 2H); 6.05 (s broad, 2H); 7.27 (m, 2H); 7.40 (m, 1 H); 8.14 (dd, 1 H); 8.27 (s, 1 H); MS (ESI+): m/z = 197.2 [M+H]+.
References:
WO2016/1389,2016,A1 Location in patent:Page/Page column 48
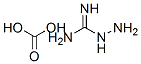
2582-30-1
383 suppliers
$19.00/100g

89-98-5
643 suppliers
$16.00/25g
![2-[(2-chlorophenyl)methylideneamino]guanidine](/CAS/20180702/GIF/13098-73-2.gif)
13098-73-2
22 suppliers
$45.00/10mg