
2-(2-cyanophenyl)acetaMide synthesis
- Product Name:2-(2-cyanophenyl)acetaMide
- CAS Number:117197-16-7
- Molecular formula:C9H8N2O
- Molecular Weight:160.1726
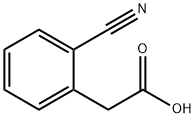
18698-99-2
139 suppliers
$8.00/250mg

117197-16-7
3 suppliers
inquiry
Yield:117197-16-7 69%
Reaction Conditions:
Stage #1: 2-(2-cyanophenyl)acetic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 1.16667 h;Inert atmosphere;
Stage #2: with ammonia in tetrahydrofuran;water at 0 - 20; for 1 h;
Steps:
7 Example 7: 2-(2-Cyanophenyl)acetamide (Ill-b)
Compound V (5.00 g, 0.031 mol) was dissolved in dry dichloromethane (150 mL). Afterwards, oxalylchloride (5.25 mL, 0.062 mol) was added, followed by a catalytic amount of Ν,Ν-dimethylformamide (about 0.05 mL). The mixture was then stirred at room temperature under an inert atmosphere for 70 minutes and evaporated to dryness. The oily residue was dissolved in dry tetrahydrofuran (THF) (50 mL) and added to an aqueous ammonia (26%, 100 mL) cooled in an ice-water bath. The mixture was stirred at 0-5 °C for 20 minutes and then additional 40 minutes at room temperature. After the reaction was completed, the mixture was poured into a separation funnel and extracted by EtOAc (2 x 250 mL). The combined organic extracts were washed with saturated NaHC03 solution (2 x 100 mL), dried over MgS04 and evaporated to dryness. The crude product was crystallized from z'PrOH/hexane to give the title compound as an off- white solid (3.41 g, 69%).
References:
WO2015/13520,2015,A1 Location in patent:Page/Page column 20-21
3759-28-2
167 suppliers
$7.00/1g

117197-16-7
3 suppliers
inquiry

55086-26-5
0 suppliers
inquiry

117197-16-7
3 suppliers
inquiry

3342-78-7
105 suppliers
$68.09/250mg

117197-16-7
3 suppliers
inquiry