
2,2-DIBROMO-1-METHYL-CYCLOPROPANECARBOXYLIC ACID synthesis
- Product Name:2,2-DIBROMO-1-METHYL-CYCLOPROPANECARBOXYLIC ACID
- CAS Number:5365-21-9
- Molecular formula:C5H6Br2O2
- Molecular Weight:257.91
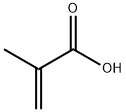
79-41-4
491 suppliers
$14.00/5g
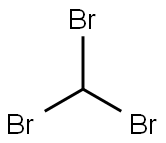
75-25-2
331 suppliers
$16.00/50g

5365-21-9
21 suppliers
$85.00/250mg
Yield:5365-21-9 93.6%
Reaction Conditions:
with benzyltriethylammonium bromide;sodium hydroxide at 20; for 4 h;
Steps:
2.1
General procedure: To a 500 ml three-neck round bottom flask equipped with a mechanical stirrer,25.8 g of methacrylic acid (0.3 mol),100 ml of chloroform,0.5 g of triethylbenzylammonium bromide,160 ml of a 30% solution of sodium hydroxide,Followed by stirring at room temperature for 4 hours.Stirring was stopped,Layers are placed.The lower layer is organic phase,The upper water layer,The aqueous phase was added 80 ml of concentrated hydrochloric acid dropwise to pH = 1 with stirring,And then stir for half an hour,Extracted with methylene chloride,The organic phases were combined,Dried, evaporated to remove the solvent,To give 43.7 g of 2,2-dichloro-1-methylcyclopropylcarboxylic acid,The yield was 86.2%Purity & gt; 95%. Instead of chloroform with tribromomethane,Using the same procedure,2,2-dibromo-1-methylcyclopropylcarboxylic acid,The yield was 93.6%Purity & gt; 95%.
References:
CN104447293,2016,B Location in patent:Paragraph 0045; 0046
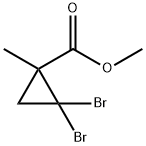
39647-01-3
4 suppliers
inquiry

5365-21-9
21 suppliers
$85.00/250mg
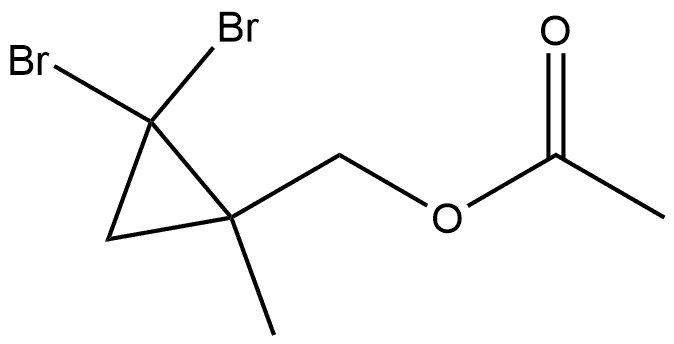
219478-95-2
0 suppliers
inquiry

5365-21-9
21 suppliers
$85.00/250mg

60288-34-8
0 suppliers
inquiry

5365-21-9
21 suppliers
$85.00/250mg

64670-28-6
5 suppliers
inquiry

5365-21-9
21 suppliers
$85.00/250mg