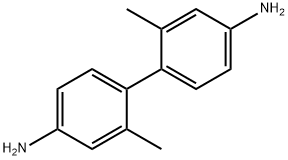
m-Tolidine synthesis
- Product Name:m-Tolidine
- CAS Number:84-67-3
- Molecular formula:C14H16N2
- Molecular Weight:212.29
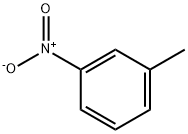
99-08-1
205 suppliers
$5.00/5G
5580-64-3
3 suppliers
inquiry
Yield:5580-64-3 79.2%
Reaction Conditions:
with 1,4-dihydroxy-9,10-anthracenedione;platinum on activated charcoal;hydrogen;sodium hydroxide in 5,5-dimethyl-1,3-cyclohexadiene;water at 65 - 75; under 6750.68 Torr; for 8 h;Autoclave;Solvent;Reagent/catalyst;Pressure;Temperature;
Steps:
1-6; 7.1; 7.2; 8-12; 1-5 The method for preparing 4,4'-diamino-2,2'-dimethyl-1,1'-biphenyl in this embodiment is as follows:
Add 205.8g of m-nitrotoluene (1.5mol) and 1.5L of xylene to the autoclave, and then add 60mL of sodium hydroxide aqueous solution (concentration: 20wt%), 6g of Pt / C catalyst (5wt%), 0.6 g of 1,4-dihydroxyanthraquinone and 1.2 g of sodium dioctyl succinate.First replace the air in the kettle with nitrogen three times, then fill with hydrogen to a pressure of 0.9 ± 0.1 MPa, first raise the temperature to 65 ± 1 ° C and hold the reaction for 5h, and then raise the temperature to 75 ± 1 ° C for 3h.After the reaction was completed, the temperature was lowered to 50-55 ° C, filtered, the catalyst was recovered and applied, and the filtrate was separated into layers. The organic layer obtained in step was cooled to 0 to 5 ° C, and 360 g of 60 wt% dilute sulfuric acid was added dropwise, and the reaction was kept at 4 to 8 ° C for 12 hours after the dropwise addition.After the reaction, the organic layer was separated, and 330 g of water was added to the aqueous layer, stirred for 1 h, and filtered. The filter cake (that is, sulfate) was added to 300 g of drinking water, and 108 g of concentrated hydrochloric acid was added. Add 10g of activated carbon, decolorize by stirring for 30min, and filter. The filtrate is adjusted to pH 8.0 to 8.5 with a 30% by weight aqueous sodium hydroxide solution, crystallized, filtered, and dried to obtain 126.2g of 4,4'-diamino-2,2'-dimethylformate. -1,1'-biphenyl,The yield is 79.2%,
References:
CN110590596,2019,A Location in patent:Paragraph 0027-0042
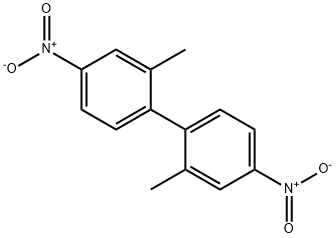
59517-20-3
1 suppliers
inquiry

5580-64-3
3 suppliers
inquiry
51437-67-3
0 suppliers
inquiry

5580-64-3
3 suppliers
inquiry
![[1,1'-Biphenyl]-4,4'-diamine, N4,N4'-bis(diphenylmethylene)-2,2'-dimethyl-](/CAS/20210305/GIF/1221066-39-2.gif)
1221066-39-2
0 suppliers
inquiry

5580-64-3
3 suppliers
inquiry
411238-29-4
0 suppliers
inquiry

5580-64-3
3 suppliers
inquiry