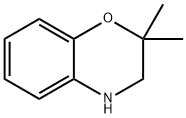
2,2-DiMethyl-3,4-dihydro-2H-1,4-benzoxazine, 97% synthesis
- Product Name:2,2-DiMethyl-3,4-dihydro-2H-1,4-benzoxazine, 97%
- CAS Number:866089-28-3
- Molecular formula:C10H13NO
- Molecular Weight:163.22
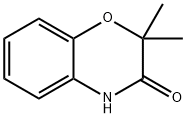
10514-70-2
36 suppliers
$34.50/250mg

866089-28-3
31 suppliers
$81.70/250mg
Yield:866089-28-3 94%
Reaction Conditions:
Stage #1: 2,2-dimethyl-2H-1,4-benzoxazine-3-onewith borane in tetrahydrofuran at 20 - 70; for 1.16667 h;
Stage #2: with hydrogenchloride in water at 50; for 0.166667 h;
Steps:
16.1
Step 1: To a solution of 2,2-dimethyl-2H-1,4-benzoxazin-3(4H)-one4 (2.658 g, 15.0 mmol) in tetrahydrofuran (10 mL) under nitrogen was added slowly a solution of borane (1.0 M in tetrahydrofuran, 22.5 mL, 22.5 mmol) via a syringe. The resulting mixture was stirred at room temperature for 10 minutes and then at 70° C. for 1 hour. After cooling, the reaction mixture was quenched with methanol (3 mL) slowly. All volatiles were removed under reduced pressure. A 1 N aqueous solution of hydrochloric acid (10 mL) was added to the liquid residue and the mixture was warmed to 50° C. for 10 minutes. After cooling, the reaction mixture was made alkaline using saturated sodium bicarbonate solution (15 mL), and extracted with ethyl acetate (25 mL). The organic layer was washed with water, brine, dried (anhydrous sodium sulfate), filtered through a pad of silica gel, and concentrated under reduced pressure to yield 2.310 g (94%) of 2,2-dimethyl-3,4-dihydro-2H-1,4-benzoxazine as a brown oil. MS (ES) m/z 164.0 ([M+H]+). 4Caliendo, G.; Perissutti, E.; Santagada, V.; Fiorino, F.; Severino, B.; Bianca, R.
References:
US2005/222142,2005,A1 Location in patent:Page/Page column 25
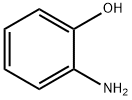
95-55-6
530 suppliers
$5.00/5G

866089-28-3
31 suppliers
$81.70/250mg
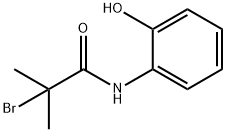
129339-29-3
3 suppliers
inquiry

866089-28-3
31 suppliers
$81.70/250mg