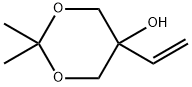
2,2-Dimethyl-5-vinyl-1,3-dioxan-5-ol synthesis
- Product Name:2,2-Dimethyl-5-vinyl-1,3-dioxan-5-ol
- CAS Number:933791-84-5
- Molecular formula:C8H14O3
- Molecular Weight:158.2
74181-34-3
135 suppliers
$13.00/100mg
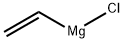
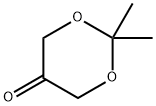
3536-96-7
208 suppliers
$67.00/100ml

933791-84-5
10 suppliers
$418.00/1g
Yield:933791-84-5 85%
Reaction Conditions:
Stage #1: vinylmagnesium chloridewith zinc(II) chloride in tetrahydrofuran;tert-butyl methyl ether at -5 - 0; for 1.25 h;
Stage #2: 2,2,-dimethyl-1,3-dioxan-5-one in tetrahydrofuran;tert-butyl methyl ether at -10 - -5; for 2.5 h;
Steps:
A RB flask was charged with solid, anhydrous ZnCl2 (0.10 mol equiv), followed by dry MTBE (8 mL/g wrt substrate) to give a slurry, which was aged at RT for 15 min and cooled to -5-O0C. A 1.6 M solution of vinyl-MgCl (1.4 mol equiv) in THF was then added over 15 min to give a light tan suspension, which was then aged for 45 min at O0C-RT and then treated with a solution of the ketone (i) (available commercially from TCI) (1 mol equiv) inMTBE (1 mL/g) over 2 hours, while maintaining the temperature between -50C - 100C. At the end of addition, the suspension was aged for additional 30 min and then slowly quenched with a 3M solution of AcOH (1.5 mol equiv) at -5°C and aged at RT for 0.5 hour to give a clear biphasic layer. Solid NaCl (6% wrt amount of H2O) was added, the mixture was stirred and the organic layer was cut. The water layer was re-extracted with MTBE (2x2mL/g) and combined with the first organic layer.The combined organic layer was successively washed with 2mL/g each of 20% aqueous NaCl, 20% aqueous Na2CO3, brine and then dried over Na2SO4, and solvent switched to CH2Cl2 to give a final concentration of 3-4 mL/g wrt to product assay. The crude solution could be used directly in the next step or purified by distillation under reduced pressure (9 torr, 80- 850C). Typical assay yield: 85-87%, typical recovery yield post distillation: 85-90%.
References:
WO2009/54887,2009,A1 Location in patent:Page/Page column 46
1826-67-1
216 suppliers
$60.89/100ml

74181-34-3
135 suppliers
$13.00/100mg

933791-84-5
10 suppliers
$418.00/1g