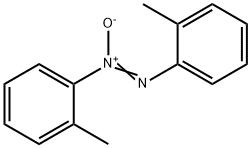
2,2'-DIMETHYLAZOXYBENZENE synthesis
- Product Name:2,2'-DIMETHYLAZOXYBENZENE
- CAS Number:956-31-0
- Molecular formula:C14H14N2O
- Molecular Weight:226.27
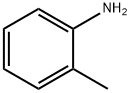
95-53-4
430 suppliers
$10.00/1g

956-31-0
9 suppliers
inquiry
Yield:956-31-0 81.5%
Reaction Conditions:
with sodium hydrogen arsenate;water;dihydrogen peroxide;Na(1+)*11H(1+)*26H2O*((RuO6)(AsW9O33)3((W6O3)(H2O)6))2(12-) in methanol; for 48 h;Irradiation;
Steps:
Methods for the Characterization of Substrates and Products
General procedure: The starting materials were commercially available and were used without further purification. The photocatalytic reactions were performed on WATTCAS Parallel Light Reactor (WP-TEC-1020HSL) with 10W COB LED. A desired amount of solid catalyst NaH11·1·53H2O was placed into the glass vessel, then a solution containing aniline, methanol, additives, H2O2 and naphthalene (an internal standard for GC-analysis) was added. The reaction vessel was sealed and stirred placed into the parallel reactor. After the reaction, the aniline oxidation products were identified with GC-MS and quantified using GC analyses with internal standard techniques. At first, 1 mmol of aniline, 2.0 mmol of H2O2, Na2HAsO4 (0.05 mmol) and 0.05 mmol% of catalyst NaH11·1·53H2O were added into 1 mL of methanol. The mixture was exposed to a 10 W white LED lamp and stirred 48 h under room temperature. Recyclability of the catalyst: the oxidation of aniline was selected as a probe reaction. Upon the completion of the reaction, the product be separated by extraction with normal hexane, then, another 1 mmol of aniline and 2.0 mmol of H2O2 were added. After that, the mixture was stirred 48 h at 10 W white LED lamp again. The process was repeatedly carried out. Reaction mixture was analyzed by GC-MS analyses. The products were isolated by column chromatography on silica gel (200-300 mesh) using petroleum ether and ethyl acetate. All compounds were characterized by 1H NMR, and mass spectrometry, which were consistent with those reported in related literatures. NMR spectra were determined on Bruker AVANCE NEO 500 MHz NMR spectrometer in CDCl3. 1H NMR chemical shifts were referenced to residual solvent as determined relative to CDCl3 (7.26 ppm). 1H NMR peaks were labeled as singlet (s), doublet (d), triplet (t), and multiplet (m). The coupling constants, J, are reported in Hertz (Hz). MS data were performed by GC-MS (Agilent 7890B GC/5973B MS, SE-54 capillary column) and GC calculations of yields were performed on Bruker 450-GC with a flame ionization detector equipped with a 30 m column (GsBP-1) with nitrogen as carrier gas
References:
Li, Huafeng;Yang, Mengnan;Yuan, Zelong;Sun, Yahao;Ma, Pengtao;Niu, Jingyang;Wang, Jingping [Chinese Chemical Letters,2022,vol. 33,# 10,p. 4664 - 4668] Location in patent:supporting information
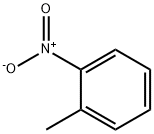
88-72-2
249 suppliers
$15.00/5g

956-31-0
9 suppliers
inquiry
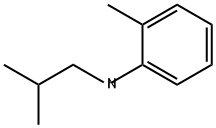
139944-70-0
0 suppliers
inquiry

956-31-0
9 suppliers
inquiry

611-23-4
33 suppliers
$218.00/5g

956-31-0
9 suppliers
inquiry
