
2,2-Dimethylpent-4-enylamine synthesis
- Product Name:2,2-Dimethylpent-4-enylamine
- CAS Number:73604-46-3
- Molecular formula:C7H15N
- Molecular Weight:113.2
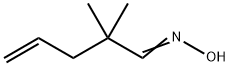
10533-71-8
0 suppliers
inquiry

73604-46-3
2 suppliers
inquiry
Yield:73604-46-3 71%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 2.41667 h;Inert atmosphere;Reflux;
Steps:
2,2-Dimethylpent-4-en-1-amine (7).3-4
To amixture of LAH (418 mg, 11.0 mmol, 2.2 equiv) in THF (11 mL) at 0 °C, was added dropwise asolution of 2,2-dimethylpent-4-enal oxime (636 mg, 5.0 mmol, 1.0 equiv) in THF (2 mL) over 15min under nitrogen. The reaction was warmed up to room temperature, allowed to stir at roomtemperature for 10 min, and then heated to reflux for 2 h. Next the reaction was cooled down to 0°C, and quenched with H2O (0.5 mL) and an aqueous solution of NaOH (0.5 mL, 15%). Themixture was allowed to stir for another 1 h, and then was diluted with H2O (50 mL) and filtered.The filtrate was extracted with Et2O (4 × 25 mL). The organic layers were combined andextracted with an aqueous solution of HCl (4 × 20 mL, 0.5 M). The aqueous layers werecombined, basified with an aqueous solution of NaOH, and then extracted with Et2O (3 × 30mL). The organic layers were combined, washed with brine (2 × 25 mL), dried over Na2SO4, andfiltered. The filtrate was concentrated in vacuo to produce 7 as a clear liquid (403 mg, 71%).
References:
Ortiz, Gerardo X.;Chansaenpak, Kantapat;Wang, Mengzhe;Ma, Xiaofen;Wang, Hui;Li, Zibo;Wang, Qiu [Synlett,2018,vol. 29,# 4,p. 410 - 414] Location in patent:supporting information
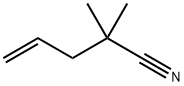
2978-30-5
15 suppliers
inquiry

73604-46-3
2 suppliers
inquiry
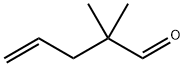
5497-67-6
82 suppliers
$45.00/50mg

73604-46-3
2 suppliers
inquiry

150675-15-3
0 suppliers
inquiry

73604-46-3
2 suppliers
inquiry

102457-33-0
0 suppliers
inquiry

73604-46-3
2 suppliers
inquiry