
2,2-Dimethylpentane synthesis
- Product Name:2,2-Dimethylpentane
- CAS Number:590-35-2
- Molecular formula:C7H16
- Molecular Weight:100.2
Yield:-
Reaction Conditions:
at 120 - 180; under 760.051 Torr;Conversion of starting material;in hydrogen;
Steps:
2 Example 2
The catalysts of Example 1 were prepared as described above in Example 1. Also, reference catalysts were prepared as described in Example 1 but with the addition of the modifier step being omitted from the preparation. Approximately 95 mg of each sample was loaded into a multi-unit reactor assay. The catalysts were pretreated in air at 450° C. for 6 hours and reduced at 200° C. in H2 for 1 hour. n-Heptane, 8 mol-%, in hydrogen was then passed over the samples at 120° C., 150° C., and 180° C., approximately 1 atm, and 0.3, 0.6, and 1.2 hr-1 WHSV (based on heptane only). The products were analyzed using online gas chromatographs. [0046] To exemplify the data, selected results are shown in FIGS. 1-4 for experiments at 180° C., 1.2 hr-1 WHSV, and using catalysts comprising 15 mass-% W, 0.5 mass-% Pt. The identity of the modifier (or first component), the amount of modifier, and the calcination temperature are identified along the x-axis of the plots of FIGS. 1-4. Data where the identity of the modifier is listed as “none”, corresponds to a reference catalyst containing no modifier. FIG. 1 is a plot of the conversion of heptane achieved by each of the catalysts. All of the catalysts indicate activity, with ytterbium, yttrium, and holmium showing the greatest conversion. It is surprising, however, that many of the catalysts demonstrate the greatest conversion when calcined at 800° C. The more expected trend would be a decreasing conversion trend with increasing calcination temperatures. A number of the catalysts of the present invention however, exhibit greater conversion with a calcination at 800° C. than at either 750° C. or 850° C. Therefore, a preferred calcination temperature is at 800° C. [0047] FIG. 2 shows the selectivity of the catalysts for C7 isomerization. This plot demonstrates that even though some cracking is occurring, selectivities to C7 isomerization remain high. FIG. 3 shows the selectivity of the catalysts for C7 isomerization to produce two of the desired dimethyl-branched isomers, 2,2-dimethylpentane and 2,4-dimethylpentane. Again, the data demonstrates that ytterbium and yttrium show superior results and are therefore, preferred modifiers. FIG. 4 shows the yield of the catalysts for C7 isomerization to produce two desired dimethyl-branched isomers, 2,2-dimethylpentane and 2,4-dimethylpentane. [0048] The data discussed above indicates a particular preference for ytterbium as a modifier. Therefore, FIGS. 5-8 present further selected results of the experiment where ytterbium was the modifier. On each of FIGS. 5-8, the amount of tungsten on the catalyst, the amount of modifier on the catalyst, the calcination temperature of the catalyst, and the weight hourly space velocity of the run is found on the x-axis. In FIG. 5, the y-axis is the conversion of the n-heptane feed. The plot demonstrates that conversion increases as the amount of tungsten increases, but conversion decreases as the weight hourly space velocity increases. However, with other variables remaining constant, increasing the amount of modifier from 2.5 mass-% to 5 mass-% does not have a dramatic effect on the conversion. FIG. 6 demonstrates that the selectivity for C7 isomerization increases with increasing space velocity. However, FIG. 7 shows that the opposite is true when considering the selectivity to two of the desired dimethyl-branched isomers, 2,2-dimethylpentane and 2,4-dimethylpentane. FIG. 8 shows the yield of two of the desired dimethyl-branched isomers, 2,2-dimethylpentane and 2,4-dimethylpentane. In general, the results in FIG. 8 indicate that the yield to the specific dimethylpentane isomers (1) decreases as the space velocity is increased; (2) is highest with a calcination temperature of 800° C.; and (3) is greater at the lower levels of modifiers.
References:
Gillespie, Ralph D. US2004/249231, 2004, A1 Location in patent:Page 6
142-82-5
581 suppliers
$16.00/25ML

108-08-7
80 suppliers
$47.29/2g

464-06-2
96 suppliers
$23.50/1g

590-35-2
75 suppliers
$34.00/1mL
82166-21-0
0 suppliers
inquiry
591-76-4
78 suppliers
$85.00/5mL
589-34-4
81 suppliers
$95.79/5g
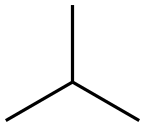
75-28-5
132 suppliers
$40.00/1g
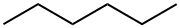
110-54-3
827 suppliers
$20.00/100ml

590-35-2
75 suppliers
$34.00/1mL
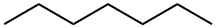
142-82-5
581 suppliers
$16.00/25ML
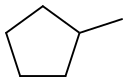
96-37-7
158 suppliers
$10.00/1g
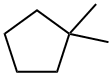
1638-26-2
32 suppliers
$1320.00/250mg

1640-89-7
46 suppliers
$45.00/10mg
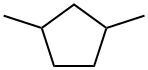
2453-00-1
24 suppliers
inquiry
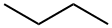
106-97-8
114 suppliers
$36.00/1mL

33429-72-0
0 suppliers
inquiry

590-35-2
75 suppliers
$34.00/1mL
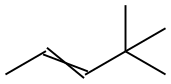
26232-98-4
11 suppliers
inquiry

590-35-2
75 suppliers
$34.00/1mL