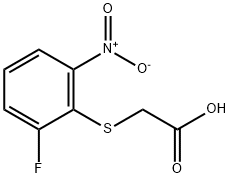
2-[(2-Fluoro-6-nitrophenyl)sulfanyl]acetic acid synthesis
- Product Name:2-[(2-Fluoro-6-nitrophenyl)sulfanyl]acetic acid
- CAS Number:1247021-61-9
- Molecular formula:C8H6FNO4S
- Molecular Weight:231.2
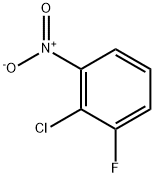
21397-07-9
132 suppliers
$30.00/1g
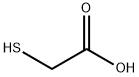
68-11-1
409 suppliers
$12.00/25g
![2-[(2-Fluoro-6-nitrophenyl)sulfanyl]acetic acid](/CAS/GIF/1247021-61-9.gif)
1247021-61-9
6 suppliers
inquiry
Yield: 95%
Reaction Conditions:
Stage #1:2-chloro-3-fluoro-nitrobenzene;mercaptoacetic acid with triethylamine in water;acetone at 32; for 1 h;Inert atmosphere;Darkness;
Stage #2: with hydrogenchloride in water; pH=4
Steps:
D.1.a
Method D; Example D-1; 4-{r4-(4-cvano-2-nnethylbenzyl)-8-fluoro-3-oxo-3,4-dihvdro-2/-/-1 ,4-benzothiazin-2- vlimethvDbenzoic acid; Preparation of D-1 -a r(2-fluoro-6-nitrophenyl)sulfanyl1acetic acid; To a mixture of 1 -chloro-2-difluoro-6-nitrobenzene (9.83g, 56 mmol) in 63ml_ aq. acetone (1 water : 2 acetone) and thethylamine (79.4ml_, 570mmol), 2-mercaptoacetic acid (5.06 ml_, 72.8 mmol, 70% aq. w/w) was added. The reaction mixture was stirred under nitrogen at 32 C in dark for 1 h. The reaction mixture was diluted with dichloromethane and the washed with water (4x50ml_). The aqueous layer was acidified to pH=4 using 2N hydrogen chloride then was extracted with ethyl acetate (2x50ml_). The combined organics were washed with saturated aqueous sodium chloride, dried (anhydrous magnesium sulfate), filtered, and concentrated to provide the title compound (12.59g, 95% yield). 1H NMR (400 MHz, DMSO-c/6) δ ppm 12.82 (br. S., 1 H) 7.83 (d, J=7.07 Hz, 1 H) 7.50 - 7.71 (m, 2 H) 7.64 (dq, J=8.34, 8.08 Hz, 1 H) 3.75 (s, 2H). HRMS Calcd for C8H6FNO4S (M+Na): 253.9894 Found: 253.9898.
References:
PFIZER INC.;CAMERON, Kimberly O'Keefe;KRAUSS, Achim Hans-Peter;LEFKER, Bruce Allen;NAIR, Sajiv Krishnan;PRASANNA, Ganesh;RUI, Eugene Yuanjin WO2010/116270, 2010, A1 Location in patent:Page/Page column 39-40