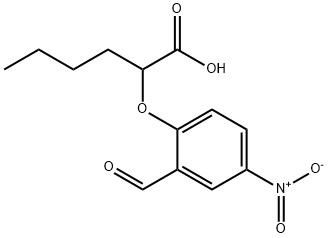
2-(2-forMyl-4-nitrophenoxy)hexanoic acid synthesis
- Product Name:2-(2-forMyl-4-nitrophenoxy)hexanoic acid
- CAS Number:335153-21-4
- Molecular formula:C13H15NO6
- Molecular Weight:281.26
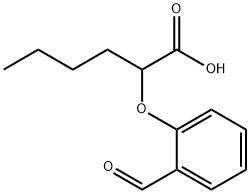
138320-27-1
31 suppliers
inquiry

335153-21-4
27 suppliers
inquiry
Yield:335153-21-4 95%
Reaction Conditions:
Stage #1: 2-(2-formyl-phenoxy)-hexanoic acidwith sulfuric acid at 5; for 0.25 h;
Stage #2: at 5; for 2 h;Product distribution / selectivity;
Steps:
4 Example 4, Preparation of 2-(2-formyl-4-nitro-phenoxy)-hexanoic acid
Example 4Preparation of 2-(2-formyl-4-nitro-phenoxy)-hexanoic acid [00335] 2-(2-formyl-4-nitro-phenoxy)-hexanoic acid can be prepared as follows: [00336] 123 g of 96% concentrated sulphuric acid was charged into a 250 ml four-neck reactor provided with a Teflon half moon paddle stirrer, a thermometer, a 50 ml dropping funnel, a cooling coil and a nitrogen inlet. [00337] The reaction medium was cooled to a temperature of close to 5° C. then 28.4 g (0.12 mole) of 2-(2-formylphenoxy)-hexanoic acid was added at the same temperature. [00338] After stirring for 15 minutes, 15.9 g (0.126 mole) of nitrating mixture (50/50) was added over 2 hours, keeping the reaction medium close to 5° C., then 76.9 g of ice was added over 30 minutes, leading to an H2SO4 titre of 60%. [00339] The reaction mixture was filtered through a no3 frit. [00340] The solid obtained was dissolved in 100 ml of dichloromethane and washed with twice 50 ml of water. [00341] The decanted organic phase was concentrated in a rotary evaporator at 20° C. to 70° C. in 20 mm of mercury (duration: 2 hours). [00342] 32.1 g of a beige yellow solid product was obtained, giving a yield of methyl 2-(2-formyl-4-nitro-phenoxy)-hexanoate of 95%, titrating at 97.0% by gas chromatography. [00343] It had the following NMR spectrum: [00344] 1H NMR (DMSO-d6): δ0.91 (t, 3H, CH3); 1.38 (m, 2H, CH2-CH3); 1.51 (m, 2H, CH2-CH2-CH3); 2.02 (m, 2H, CH2-CH); 5.24 (t, 1H, CH); 7.34 (d, J=9Hz, 1H, ArH); 8.44 (d, J=2 Hz, 1H, ArH); 8.47 (dd, J=9 Hz, J=2 Hz, 1H, ArH); 10.42 (s, 1H, CHO); 13.45 (broad peak, 1H, COOH).
References:
US6855842,2005,B1 Location in patent:Page/Page column 16-17

335153-23-6
23 suppliers
inquiry

335153-21-4
27 suppliers
inquiry
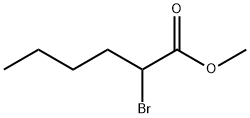
5445-19-2
246 suppliers
$16.00/25g

335153-21-4
27 suppliers
inquiry

97-51-8
413 suppliers
$6.00/5g

335153-21-4
27 suppliers
inquiry
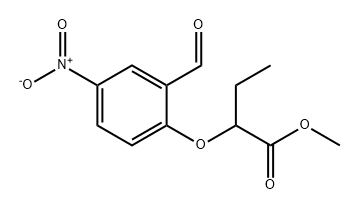
847869-79-8
0 suppliers
inquiry

335153-21-4
27 suppliers
inquiry