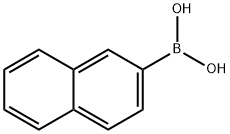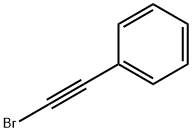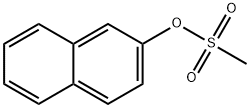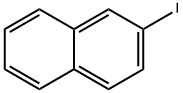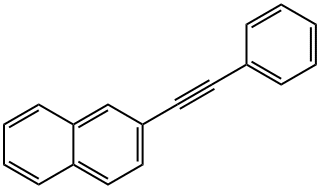
2-(2-phenylethynyl)naphthalene synthesis
- Product Name:2-(2-phenylethynyl)naphthalene
- CAS Number:23975-17-9
- Molecular formula:C18H12
- Molecular Weight:228.29
Yield:23975-17-9 98%
Reaction Conditions:
with copper(l) iodide;8-quinolinol;sodium phosphate in ethanol at 80; for 24 h;Suzuki coupling;
Steps:
4.2. General procedure for CuI-catalyzed Suzuki coupling reaction of organoboronic acids with alkynyl bromides
General procedure: A 5.0 mL of reaction tube was charged with organoboron compound (1.0 mmol), alkynyl bromide (1.0 mmol), CuI (0.10 mmol), 8-hydroxyquinoline (0.20 mmol), ethanol (2.0 mmol). The mixture was stirred at 80 °C for 24 h, and then washed with ethyl acetate (3.0 mL×3), the combined ethyl acetate was concentrated under reduced pressure. The obtained residue was purified by flash column chromatography on silica gel (petroleum ether as eluting agent) to give the corresponding pure cross-coupling product.
References:
Wang, Shihua;Wang, Min;Wang, Lei;Wang, Bo;Li, Pinhua;Yang, Jin [Tetrahedron,2011,vol. 67,# 26,p. 4800 - 4806] Location in patent:experimental part