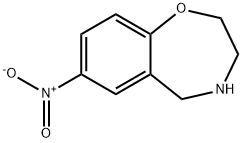
2,3,4,5-Tetrahydro-7-nitro-1,4-benzoxapine synthesis
- Product Name:2,3,4,5-Tetrahydro-7-nitro-1,4-benzoxapine
- CAS Number:216008-29-6
- Molecular formula:C9H10N2O3
- Molecular Weight:194.19
21228-43-3
10 suppliers
inquiry

216008-29-6
8 suppliers
inquiry
Yield:216008-29-6 96%
Reaction Conditions:
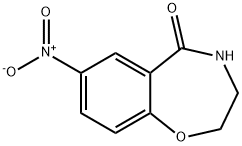
Stage #1: 7-nitro-3,4-dihydro-2H-benzo[f][1,4]oxazepin-5-onewith borane in tetrahydrofuran; for 4 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0; for 1 h;Heating / reflux;
Steps:
9.iv
7-Nitro-3,4-dihydro-l,4-benzoxazepin-5(2H)-one (0.99 g, 4.7 mmol) was suspended in TΗF (7 ml) and BH3 (1 M in THF, 19 ml, 19 mmol) was added. The mixture was heated at reflux for 4 h. The mixture was cooled to O0C and hydrochloric acid (4 M, 7 ml) was added. The mixture was heated at reflux for 1 h and then concentrated by evaporation. The residue was diluted with water (30 ml) and neutralized with solid sodium hydrogen carbonate. The mixture was extracted with EtOAc (3x). The organic phase was dried EPO
References:
WO2007/4959,2007,A1 Location in patent:Page/Page column 35-36