
2,3,4,9-TETRAHYDRO-1H-CARBAZOL-1-OL synthesis
- Product Name:2,3,4,9-TETRAHYDRO-1H-CARBAZOL-1-OL
- CAS Number:1592-62-7
- Molecular formula:C12H13NO
- Molecular Weight:187.24
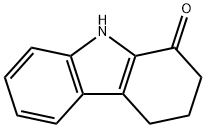
3456-99-3
57 suppliers
$92.00/500mg

1592-62-7
27 suppliers
inquiry
Yield:1592-62-7 90%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran;methanol at 20; for 2 h;Schlenk technique;Inert atmosphere;
Steps:
13. General Procedure I: Preparation of Racemic Alcohols
General procedure: An oven dried Schlenk tube that was capped with a glass stopper and equipped with a magnetic stirring barwas charged with a tetrahydrocarbazol-1-one or tetrahydrocarbazol-4-one (1.0 mmol, 1.00 equiv) derivative.The Schlenk tube was evacuated for 15 minutes, back-filled with dry N2, and the glass stopper was replacedwith a rubber septum under positive pressure of dry nitrogen. Solid particles were dissolved by adding dryTHF (4 mL) and dry MeOH (2 mL). Then, NaBH4 (1.5 mmol, 1.50 equiv) was added portionswise. Theconversion was followed by TLC. After the conversion was completed, THF and MeOH were removed byrotary evaporation under reduced pressure and the residue was mixed with distilled water (20 mL) andextracted with DCM (3 × 50 mL). The combined organic extracts were dried over Na2SO4, filtered, andconcentrated by rotary evaporation in vacuo. Purification by flash column chromatography on silica gel gaveracemic alcohols.
References:
Dilek, ?mer;Patir, Süleyman;Ertürk, Erkan [Synlett,2019,vol. 30,# 1,art. no. ST-2018-U0690-L,p. 69 - 72] Location in patent:supporting information
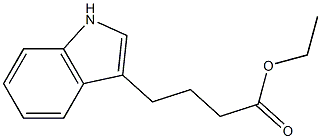
49850-32-0
5 suppliers
inquiry

1592-62-7
27 suppliers
inquiry
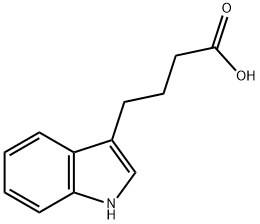
133-32-4
644 suppliers
$10.00/5g

1592-62-7
27 suppliers
inquiry
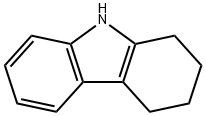
942-01-8
201 suppliers
$7.00/5g

1592-62-7
27 suppliers
inquiry
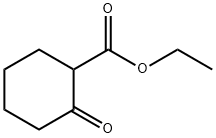
1655-07-8
301 suppliers
$6.00/5g

1592-62-7
27 suppliers
inquiry