
2-(3,4-DIFLUOROPHENYL)PROPAN-2-AMINE synthesis
- Product Name:2-(3,4-DIFLUOROPHENYL)PROPAN-2-AMINE
- CAS Number:306761-17-1
- Molecular formula:C9H11F2N
- Molecular Weight:171.19
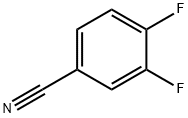
64248-62-0
424 suppliers
$6.00/5g
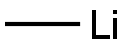
917-54-4
173 suppliers
$52.10/25ml

306761-17-1
26 suppliers
$45.00/50mg
Yield:306761-17-1 51%
Reaction Conditions:
with cerium(III) chloride in tetrahydrofuran;diethyl ether at -25; for 1 h;
Steps:
16 4.1.16 2-(3,4-Difluorophenyl)propan-2-amine (20)
A solution of anhydrous CeCl3 (2.84g, 11.5mmol) in THF (18mL) was stirred at 45°C for 3h and cooled down to room temperature. Then, 3,4-difluorobenzonitrile (800mg, 5.75mmol) was added and the mixture was cooled down to-25°C before addition of MeLi (9.6mL, 14.4mmol, 1.5M in Et2O). The solution was stirred 1hat-25°C and quenched with NaOH 30% (4mL). The mixture was stirred for 16hat room temperature and the cerium salts were filtered and washed with THF. The filtrate was dried over MgSO4 and concentrated under reduced pressure. The residue was dissolved in THF and HCl (4N in dioxane) was added and the solution was concentrated in vacuo. The resulting salts were filtered, washed with hexanes and then treated with aqueous ammonium hydroxide (5mL). The solution was then extracted with CH2Cl2, dried over MgSO4 and concentrated in vacuo. The residue was purified by silica gel column chromatography using CH2Cl2/ MeOH (98:2) to afford 20 (502mg, 51%). 1H NMR (400MHz, Chloroform-d) δ 7.41-7.32 (m, 1H), 7.27-7.19 (m, 1H), 7.11 (dt, J=10.2, 8.4Hz, 1H), 1.49 (s, 6H). 13C NMR (101MHz, Chloroform-d) δ 150.51 (dd, J=121.6, 12.6Hz), 148.83-147.43 (m), 147.48 (d, J=16.9Hz), 120.69 (dd, J=6.1, 3.5Hz), 116.62 (d, J=16.8Hz), 114.26 (d, J=17.8Hz), 52.12 (d, J=1.4Hz), 33.01. 19F NMR (377MHz, Chloroform-d) δ-139.4 to-139.5 (m),-143.4 to-143.5 (m). HRMS (ESI): m/z [M+H]+ calcd for C9H12F2N: 172.0938, found: 172.0932.
References:
Sari, Ozkan;Boucle, Sebastien;Cox, Bryan D.;Ozturk, Tugba;Russell, Olivia Ollinger;Bassit, Leda;Amblard, Franck;Schinazi, Raymond F. [European Journal of Medicinal Chemistry,2017,vol. 138,p. 407 - 421]