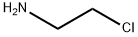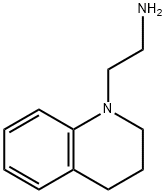
2-(3,4-DIHYDRO-2H-QUINOLIN-1-YL)-ETHYLAMINE synthesis
- Product Name:2-(3,4-DIHYDRO-2H-QUINOLIN-1-YL)-ETHYLAMINE
- CAS Number:37481-18-8
- Molecular formula:C11H16N2
- Molecular Weight:176.26
635-46-1
352 suppliers
$5.00/10g
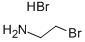
2576-47-8
470 suppliers
$8.00/25g

37481-18-8
56 suppliers
$82.50/250mg
Yield:37481-18-8 79%
Reaction Conditions:
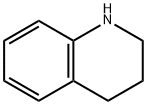
Stage #1: 1,2,3,4-tetrahydroisoquinoline;2-bromoethylamine hydrobromidewith sodium hydrogencarbonate in water at 95; for 3.75 h;Reflux;
Stage #2: with sodium hydroxide in water at 20;
Steps:
Aminoalkylation of NH-substrates upon treatment with 2-haloethyl- or 3-halopropylamine salts. Method A. Synthesis of diamines 2-5 and 7-11.
General procedure: A mixture of the corresponding NH-substrate (0.415 mol) (see Table 1), 2- or 3-haloalkylamine hydrohalide and water (100 mL) was heated to 95 ° C (in a bath) with stirring, then NaHCO3 was added in small portion. After stirring for ~15 min at 95 ° C, the reaction mixture was refluxed with stirring using a heating mantle until completion of the reaction. The amount of hydrohalide and NaHCO3 , as well as the time of heating are given in Table 1. The mixture was allowed to cool down to ~20 ° C, followed by the addition of a 40% aqueous NaOH (75 mL) and water until dissolution of the precipitate (~250 mL). The organic layer was separated, the aqueous layer was extracted with diethyl ether and CH2Cl2 (twice). The combined organic layers were washed with saturated aqueous solution of NaCl and dried with MgSO4. The solvent was evaporated in vacuo, the residue was distilled.
References:
Vasilyeva;Vorobyeva;Osipov [Russian Chemical Bulletin,2016,vol. 65,# 9,p. 2211 - 2215][Izv. Akad. Nauk, Ser. Khim.,2016,# 9,p. 2211 - 2215,5]

66487-31-8
4 suppliers
inquiry

37481-18-8
56 suppliers
$82.50/250mg
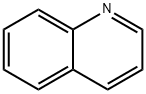
91-22-5
385 suppliers
$10.00/25g

37481-18-8
56 suppliers
$82.50/250mg