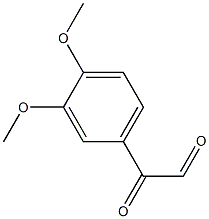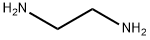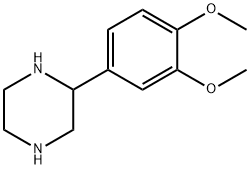
2-(3,4-DIMETHOXY-PHENYL)-PIPERAZINE synthesis
- Product Name:2-(3,4-DIMETHOXY-PHENYL)-PIPERAZINE
- CAS Number:65709-39-9
- Molecular formula:C12H18N2O2
- Molecular Weight:222.28
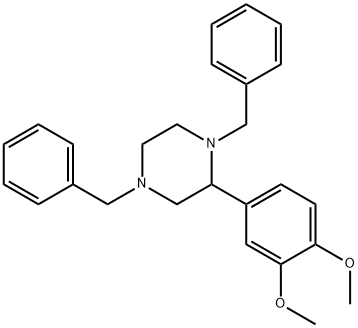
1421005-05-1
0 suppliers
inquiry

65709-39-9
12 suppliers
inquiry
Yield:65709-39-9 88%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in methanol; for 4 h;
Steps:
General procedure for the synthesis of piperazines
General procedure: Olefins 1 (1 mmol) were dissolved in dry THF (15 mL) under N2 and HgO·2HBF4 (1.1 mmol) was added portionwise followed by the addition of N,N′-disubstitutedethylene diamine 2 (1.5 mmol). The reaction mixture was stirred at 70 °C for the appropriate time as shown in Table 1. After completion of the reaction, as indicated by TLC, the reaction mixture was diluted with water and extracted with CH2Cl2 (3 × 30 mL). The combined organic layers were dried over anhydrous Na2SO4, concentrated in vacuo, and purified by flash column chromatography on silica gel to afford corresponding protected piperazine derivatives 3. The compound 3 was dissolved in MeOH (9 mL) containing acetic acid (1 mL) and then added Pd/C.27 The reaction mixture was stirred for 4 h under H2 on a Parr hydrogenator and the progress of the reaction was monitored on TLC. After completion of reaction, the catalyst was filtered off and the organic mixture was evaporated in a rota-vapour. The crude mixture was purified by column chromatography using chloroform:methanol (95:5-80:20) as eluting mixture to get the corresponding product 4. The products reported herein, were characterized by 1H NMR, 13C NMR, and mass spectroscopy. The spectroscopic data of the compounds are in agreement with those reported in the literature.29 Spectral data for 3a29a-d. 1H NMR (400 MHz, CDCl3): δ 1.92-2.14 (m, 4H), 2.14-2.66 (m, 2H), 3.82 (s, 4H), 4.14 (m, 1H), 7.01-7.49 (m, 15H); 13C NMR: δ 29.4, 36.5, 41.4, 62.5, 125.80, 127.40, 128.41, 144.70; MS (ESI): 342 (M+).
References:
Kour, Harpreet;Paul, Satya;Singh, Parvinder Pal;Gupta, Monika;Gupta, Rajive [Tetrahedron Letters,2013,vol. 54,# 8,p. 761 - 764]
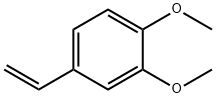
6380-23-0
81 suppliers
$30.00/1g

65709-39-9
12 suppliers
inquiry
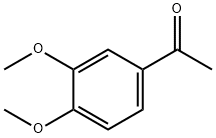
1131-62-0
318 suppliers
$14.00/5g

65709-39-9
12 suppliers
inquiry
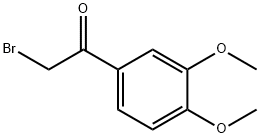
1835-02-5
107 suppliers
$7.00/1g

65709-39-9
12 suppliers
inquiry