
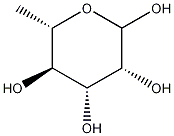
2,3,4-TRI-O-BENZYL-L-RHAMNOPYRANOSE synthesis
- Product Name:2,3,4-TRI-O-BENZYL-L-RHAMNOPYRANOSE
- CAS Number:210426-02-1
- Molecular formula:C27H30O5
- Molecular Weight:434.52
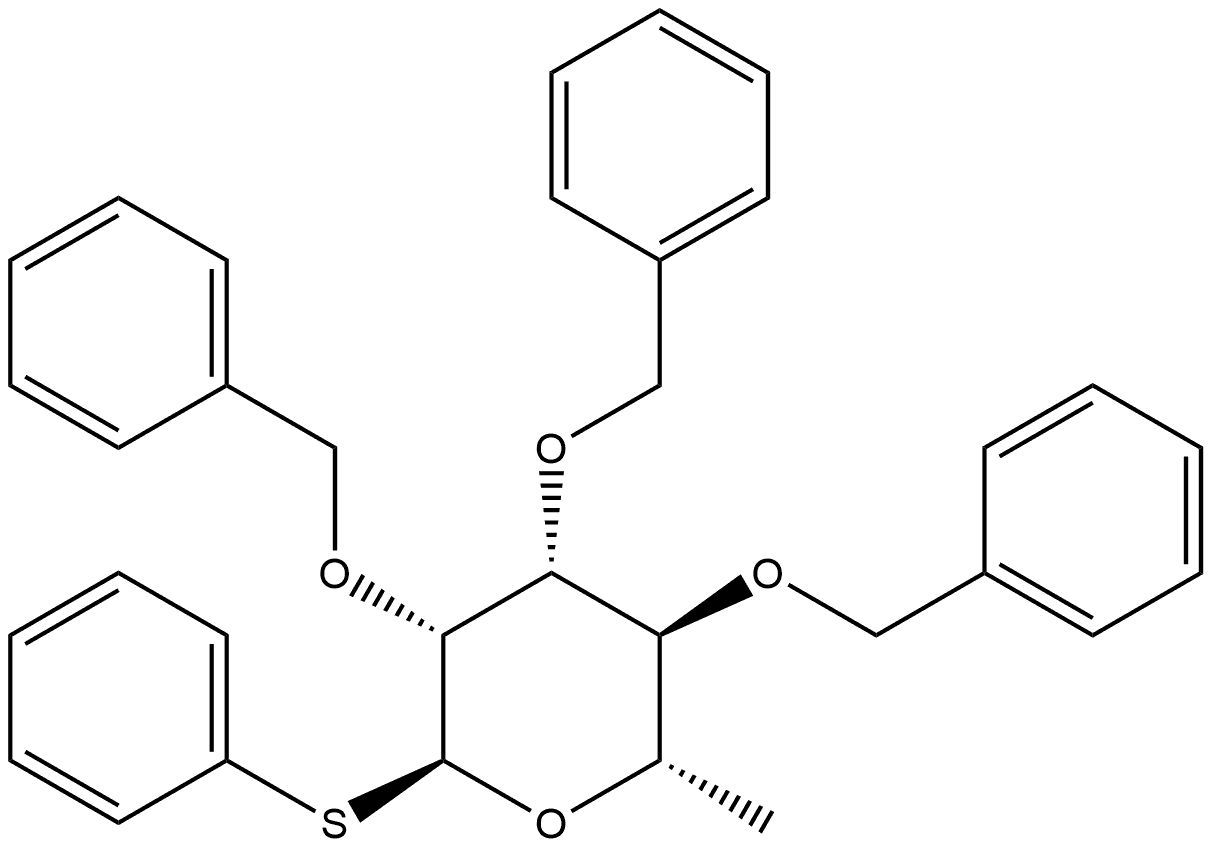
131897-04-6
0 suppliers
inquiry

210426-02-1
18 suppliers
inquiry
Yield:210426-02-1 95%
Reaction Conditions:
with N-Bromosuccinimide;water in acetonitrile at 0 - 20; for 2 h;Darkness;
Steps:
2,3,4-tri-O-benzyl-L-rhamnopyranose (20)
Following a procedure presented in literature1, compound 19 (3.24 g, 6.15 mmol) was dis-solved in a 9:1 mixture of acetonitrile:water (60 mL) at 0 °C and NBS (3.26 g, 18.45 mmol, 3 equiv.) was added in the dark. The reaction left to stir at room temperature for 120 min. when TLC analysis showed the complete conversion of the starting material to the desired product. The mixture was quenched with saturated Na2S2O3 (20 mL), concentrated in vacuo to remove acetonitrile and diluted with CH2Cl2 (100 mL). The organic phase was then washed with satu-rated NaHCO3, deionised H2O and brine. The organic layer was dried over anhydrous MgSO4, filtered off and concentrated in vacuo to give the product 20 as a yellow sirup (2.54 g, 5.86 mmol, 95 %).Rf (8:2 heptane: EtOAc) = 0.271H NMR (500 MHz, Chloroform-d) δ 7.42 - 7.27 (m, 15H, Ph), 5.17 (s, 1H, H-1), 5.12 (d, J = 11.6 Hz, 1H, CH2Ph), 4.96 (dd, J = 10.8, 7.2 Hz, 1H, CH2Ph), 4.82 - 4.56 (m, 4H, CH2Ph), 4.01 - 3.90 (m, 1H, H-3), 3.88 - 3.79 (m, 1H, H-2), 3.65 (t, J = 9.4 Hz, 1H, H-4), 3.42 - 3.33 (m, 1H, H-5), 1.35 (dd, J = 11.7, 6.1Hz, 3H, H-6). 13C NMR (126 MHz, Chloroform-d) δ 138.7 (CPh,ipso), 138.4 (CPh,ipso), 138.2 (CPh,ipso), 128.7 (CPh), 128.7 (CPh), 128.5 (CPh), 128.5 (CPh), 128.4 (CPh), 128.1 (CPh), 128.1 (CPh), 127.8 (CPh), 127.8 (CPh), 93.0 (C-1), 83.2, 80.6, 75.5, 75.2, 73.0, 72.4, 68.3, 18.2 (C-6).NMR data of the product is in accordance with literature.7 The product contains some CH2Cl2 which is visible in the spectrum.
References:
Korber, Nora Katharina;Pedersen, Christian Marcus [Carbohydrate Research,2022,vol. 511,art. no. 108497] Location in patent:supporting information
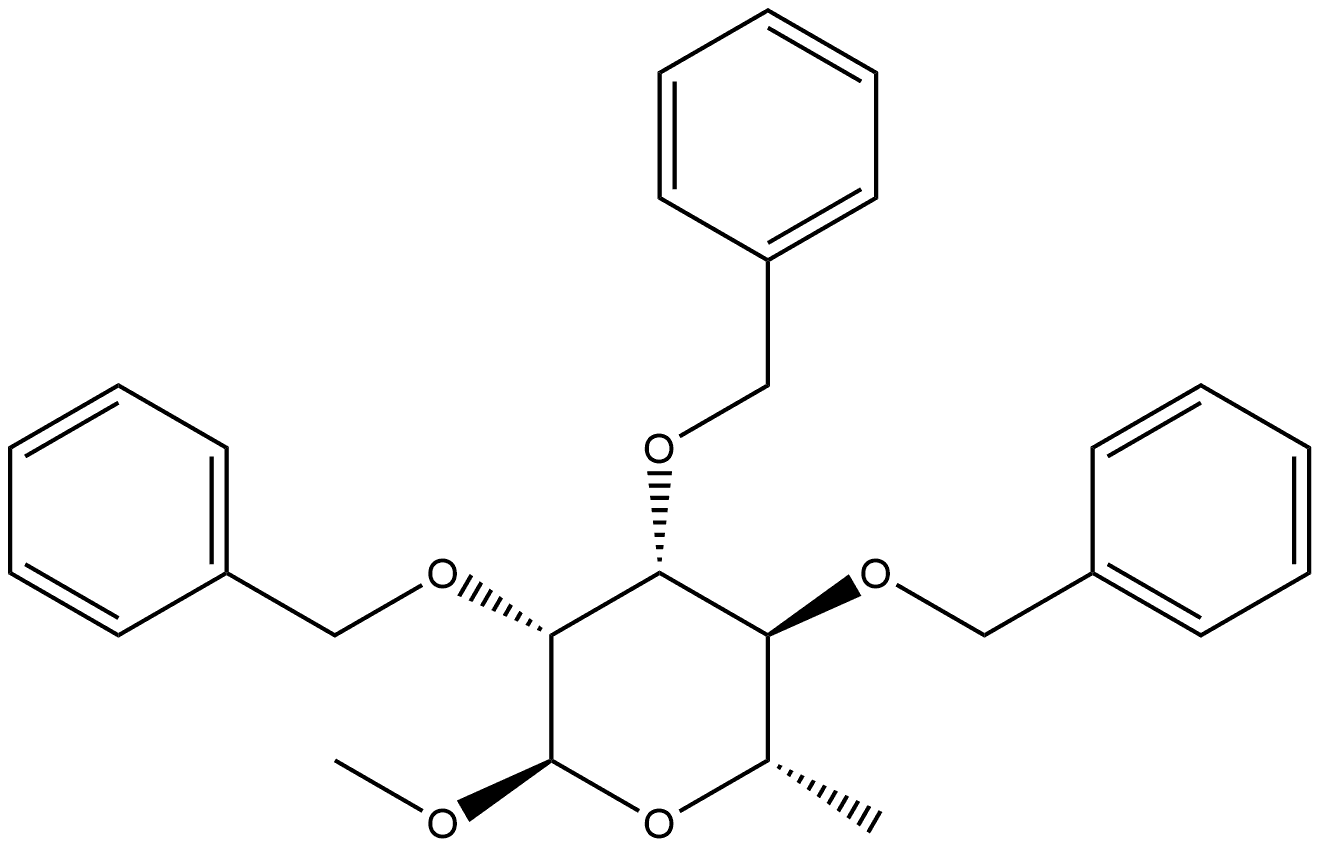
59055-57-1
0 suppliers
inquiry

210426-02-1
18 suppliers
inquiry
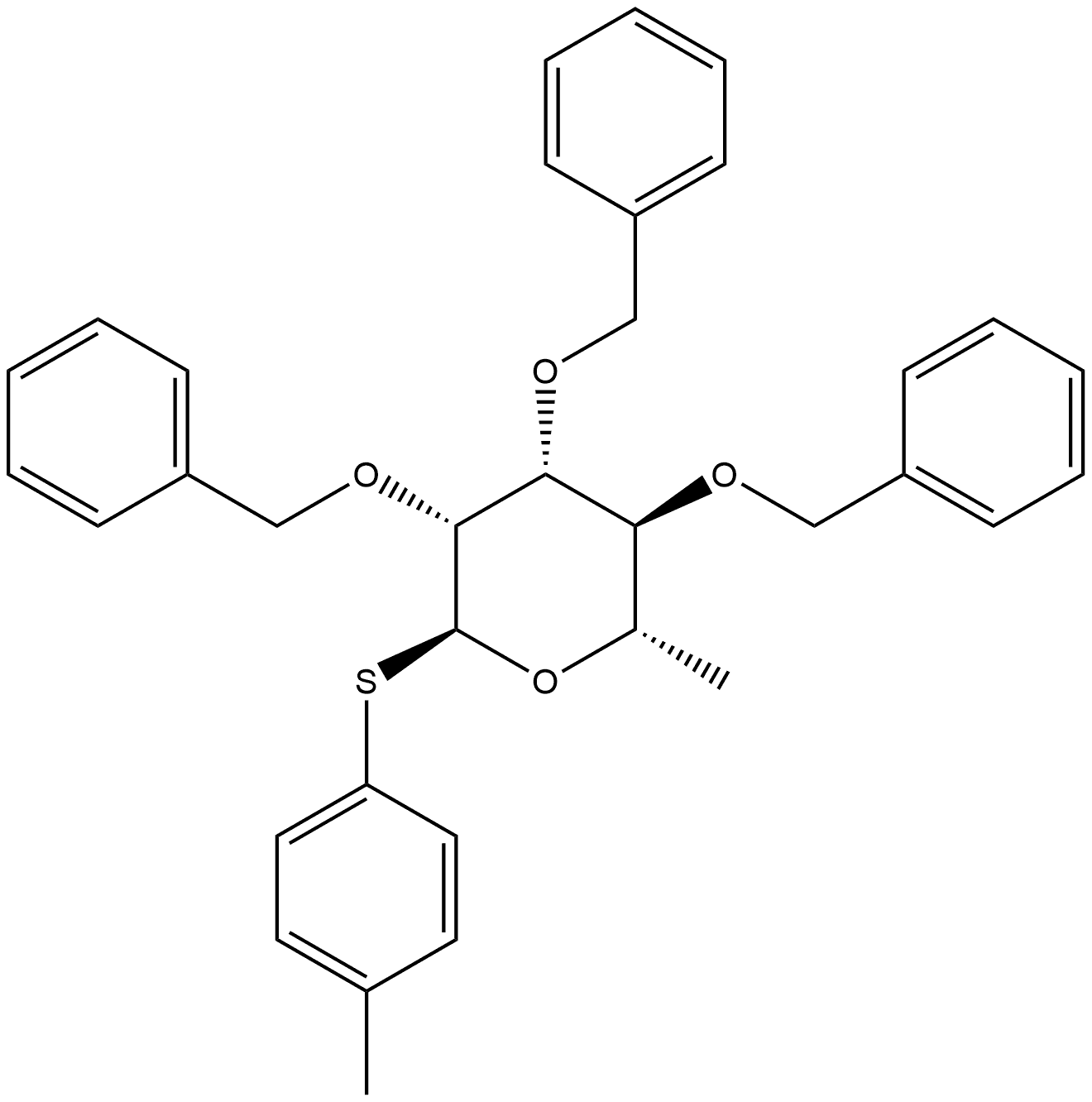
916994-68-8
0 suppliers
inquiry

210426-02-1
18 suppliers
inquiry
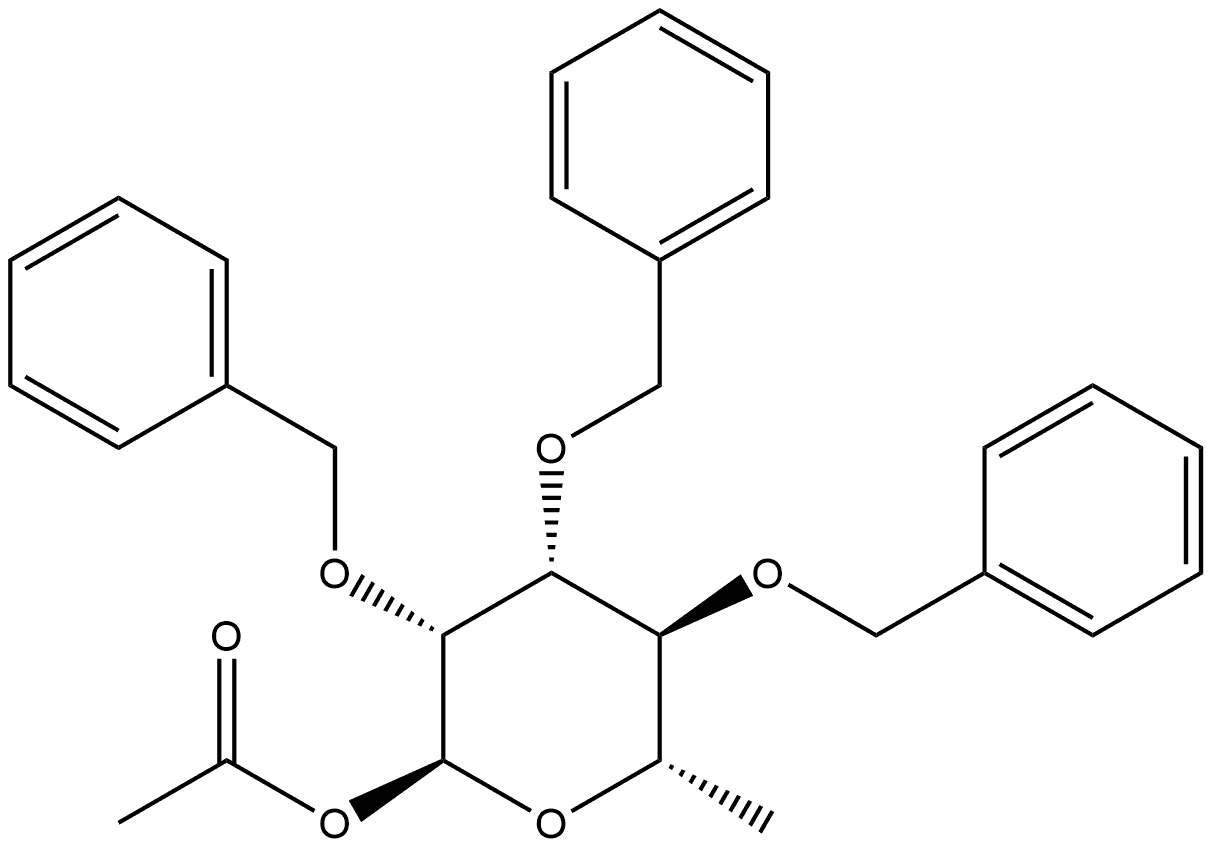
80152-99-4
0 suppliers
inquiry

210426-02-1
18 suppliers
inquiry
73-34-7
1 suppliers
inquiry

210426-02-1
18 suppliers
inquiry