
2,3,5,6-tetraiodoterephthalic acid synthesis
- Product Name:2,3,5,6-tetraiodoterephthalic acid
- CAS Number:7606-84-0
- Molecular formula:C8H2I4O4
- Molecular Weight:669.72
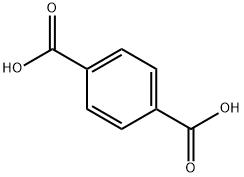
100-21-0
590 suppliers
$6.00/25g

7606-84-0
8 suppliers
inquiry
Yield:7606-84-0 45%
Reaction Conditions:
Stage #1: terephthalic acidwith sulfuric acid at 100;
Stage #2: with iodine at 125 - 190; for 7 h;Darkness;
Steps:
a (Actual) Experimental Procedure for Synthesizing Tetraiodoterephthalic Acid (TIPA):
To a round bottom flask was added terephthalic acid (2.49 g, 14.94 mmol) and 20% fuming sulfuric acid (40.00 mL, 275 mmol). The reaction flask was vented to a sulfuric acid bubbler and allowed to stir at 100° C. until the terephthalic acid dissolved (approximately 30-45 min). Iodine (10.00 g, 39.40 mmol) was added to the reaction flask and the temperature increased to 125° C. for 30 min. The reaction setup was then wrapped in foil and the temperature was increased to 150° C. for 15 min, 170° C. for 15 min, and finally 190° C. The reaction stirred at 190° C. for 6 hrs. The reaction mixture was cooled overnight. The reaction mixture was then poured into ice cold water and the flask was washed with water two times. The combined aqueous layers were vacuum filtered until a chunky pink solid was obtained. The solid was dissolved into aqueous KOH to a pH of 10 and the liquid was vacuum filtered. NaHSO3 (0.25 g, 2.40 mmol), which is a weaker acid, was added to the filtrate and the product was precipitated using concentrated hydrochloric acid to a pH of 1. The precipitate was vacuum filtered to dryness and allowed to air dry overnight on a watch glass. The light pink solid was then triturated by pulverization in acetone followed by vacuum filtration, and the remaining solid was washed with portions of acetone until the filtrate ran clear and colorless. The white solid was crushed up in hot methanol and vacuum filtered, then washed with small portions of hot methanol (approximately 2-3 washings). The white solid was dried on high vacuum to give tetraiodoterephthalic acid in 45% yield (4.54 g, 6.78 mmol). 13C NMR: δ 170, 150, 108 ppm. LCMS: 624 (MW-CO2) m/z. IR: 2900, 1703 cm-1.
References:
US10342232,2019,B1 Location in patent:Page/Page column 4; 5

100-21-0
590 suppliers
$6.00/25g
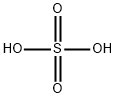
7664-93-9
0 suppliers
$22.69/1L
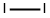
7553-56-2
652 suppliers
$20.69/25G
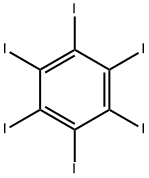
608-74-2
9 suppliers
inquiry

7606-84-0
8 suppliers
inquiry