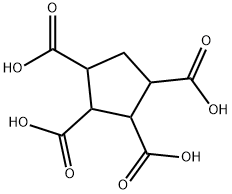
2,3,5-Tricarboxycyclopentane-1-acetic acid synthesis
- Product Name:2,3,5-Tricarboxycyclopentane-1-acetic acid
- CAS Number:24434-90-0
- Molecular formula:C10H12O8
- Molecular Weight:260.2
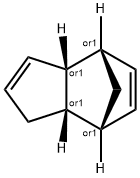
1755-01-7
8 suppliers
inquiry

24434-90-0
18 suppliers
inquiry
Yield:24434-90-0 1450 g
Reaction Conditions:
Stage #1: bi(cyclopentadiene)with sodium periodate;osmium(VIII) oxide in tetrahydrofuran;water at 20; for 20 h;Large scale;
Stage #2: with dihydrogen peroxide in propan-1-ol;water; for 25 h;Reflux;Large scale;Concentration;Time;
Steps:
8 Preparation Example 8
Preparation Example 8
1000g of dicyclopentadiene was added to 20000 ml tetrahydrofuran and 20000ml of water, mixed, was added 7000g sodium periodate, 2g osmium tetroxide, stirred for 20 hours at room temperature, poured into 200000ml water, precipitated solid was filtered. The solid was added 10000ml of propanol, 10000ml water, 1000ml 50% hydrogen peroxide, was slowly warmed to reflux for 3 hours. 500ml of 50% hydrogen peroxide was added and reacted 2 hours, then added with 500ml hydrogen peroxide, reacted for 20 hours. Solution was evaporated under reduced pressure to give a pale yellow viscous liquid 1450g, i.e., the product 2,3,5-tricarboxycyclopentylacetic acid.
References:
CN104193612,2016,B Location in patent:Paragraph 0059; 0060