
2-(3-CHLORO-4-METHOXY-PHENYL)-ETHYLAMINE synthesis
- Product Name:2-(3-CHLORO-4-METHOXY-PHENYL)-ETHYLAMINE
- CAS Number:18149-08-1
- Molecular formula:C10H15NO
- Molecular Weight:165.23

62993-57-1
0 suppliers
inquiry

18149-08-1
14 suppliers
inquiry
Yield:18149-08-1 81%
Reaction Conditions:
Stage #1: 2-methyl-4-(trans-2-nitro-vinyl)-anisolewith borane in tetrahydrofuran at 90; for 14.3333 h;
Stage #2: with water in tetrahydrofuran; for 0.5 h;
Stage #3: with hydrogenchloride;water in tetrahydrofuran; pH=2; for 5 h;
Steps:
2
Step 2.; A solution of methyl 2-methyl-4-[(E)-2-nitroethenyl]phenyl ether (7.89 g; 40.88 mmol) in dry THF (50 ml) was added to borane (245 ml of 1M solution in THF; 245 mmol), under nitrogen, dropwise over 20 minutes. The reaction mixture was then heated to 90C. After 14 hr it was cooled to rt and ice-cold water (100 m .) was added dropwise over 30 minutes. The pH was adjusted to No.2 with 1N HCl and heated to reflux for 5 hr. Upon cooling, the reaction mixture was extracted with ethyl ether (2x300 ml) and the ether phases were discarded. The pH of the aqueous phase was adjusted to 10 with 1 N and and extracted with ethyl ether (2x200 ml). The combined organic phases were dried over MgSO4, filtered and concentrated to provide 2-(4-methoxy-3-methylphenyl)ethanamine (5.46 g; 81% yield), which was used without any further purification. 'H NMR (CDOs) 8 6. 95 (d, 1H, J= 8. 2), 6. 94 (s, 1H), 6. 72 (d, 1H, J= 8. 0), 3. 8 (s, 3H), 2. 88 (t, 2H, 6. 8), 2. 62 (t, 2H, 6. 9), 2. 18 (s, 3h).
References:
WO2003/74495,2003,A1 Location in patent:Page/Page column 64
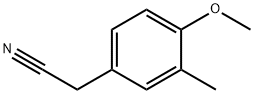
75391-57-0
51 suppliers
inquiry

18149-08-1
14 suppliers
inquiry

32723-67-4
171 suppliers
$45.00/5g

18149-08-1
14 suppliers
inquiry