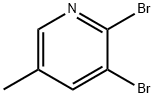
2,3-DIBROMO-5-METHYLPYRIDINE synthesis
- Product Name:2,3-DIBROMO-5-METHYLPYRIDINE
- CAS Number:29232-39-1
- Molecular formula:C6H5Br2N
- Molecular Weight:250.92
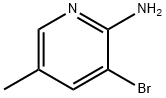
17282-00-7
329 suppliers
$5.00/1g

29232-39-1
204 suppliers
$10.00/1g
Yield: 97%
Reaction Conditions:
Stage #1:3-bromo-5-methylpyridin-2-amine with hydrogen bromide in water
Stage #2: with bromine in water at 0 - 5; for 0.166667 h;
Stage #3: with sodium hydroxide;sodium nitritemore than 3 stages;
Steps:
32.5
In a 30-ml three-necked flask, 2-amino-3-bromo-5- methylpyridine (187 mg, 1.00 mmol) ) was charged, 48% aqueous hydrogen bromide solution (1 ml) was added and 2-amino-3- bromo-5-methylpyridine was dissolved. Then, the mixture was ice-cooled, and bromine (154 μl, 3.00 mmol) was slowly added dropwise thereto at the internal temperature of 2 to 5°C. The mixture was stirred at the same temperature for 10 minutes. To this solution, sodium nitrite (174 mg, 2.5 mmol) /water (500 μl) solution was added dropwise at the same temperature, and the mixture was stirred for 1 hour. Sodium hydroxide (377 mg, 9.4 mmol) /water (2 ml) was slowly added dropwise thereto. The mixture was stirred at room temperature for 1 hour. The solution was poured into a separating funnel. The organic layer was separated by extraction using ethyl acetate (10 ml)
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED WO2008/16184, 2008, A1 Location in patent:Page/Page column 187-188
78-85-3
178 suppliers
$52.79/5ml
3252-43-5
113 suppliers
$19.00/25g

29232-39-1
204 suppliers
$10.00/1g