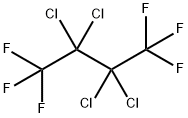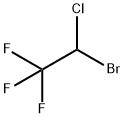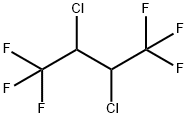
2,3-DICHLORO-1,1,1,4,4,4-HEXAFLUOROBUTANE synthesis
- Product Name:2,3-DICHLORO-1,1,1,4,4,4-HEXAFLUOROBUTANE
- CAS Number:384-54-3
- Molecular formula:C4H2Cl2F6
- Molecular Weight:234.96
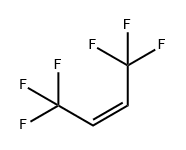
692-49-9
33 suppliers
inquiry

384-54-3
18 suppliers
$135.00/5G
Yield:-
Reaction Conditions:
with chlorine at 200; for 1 h;Gas phase;Reagent/catalyst;Temperature;
Steps:
1-5
Starting material compound: (Z)1336mzz: (Z)-1,1,1,4,4,4-hexafluoro-2-butene Target compound: HCFC-336mdd: 2,3-dichloro-1,1,1,4,4,4-hexafluorobutane (0102) The above chlorination reaction can also produce 2,2,3,3-tetrachloro-1,1,1,4,4,4-hexafluorobutane (HCFC-316maa, CF3CCl2CCl2CF3). (0103) A zeolite catalyst was placed in a SUS pipe (outer diameter: inch) used as a reaction tube. After the catalyst was dried at 500° C. for 3 hours in a nitrogen atmosphere, the temperature was reduced to a reaction temperature. First, chlorine diluted with nitrogen was allowed to flow through the reaction tube, the chlorine concentration was gradually increased, and finally, a chlorination treatment with the catalyst was performed in a 100% chlorine atmosphere. (Z)-1336mzz (starting material compound) was allowed to flow through the reaction tube at a normal pressure so that the contact time (W/F0) of (Z)-1336mzz (starting material compound) with the zeolite catalyst was 12 g*sec/cc or 24 g*sec/cc. (0104) The chlorine was used at a Cl2/alkene molar ratio of 2. (0105) The reaction was performed by a gas-phase continuous flow process. (0106) The reactor was heated at 100° C. or 200° C. to start the chlorination reaction. (0107) After one hour from the start of the chlorination reaction, the distillate that passed through an abatement tower was collected. (0108) After that, mass spectrometry was performed by gas chromatography (trade name: GC-2014, produced by Shimadzu Corporation) in accordance with gas chromatography-mass spectrometry (GC-MS), and structural analysis based on an NMR spectrum was performed using an NMR spectrometer (trade name: 400YH, produced by JEOL). (0109) The results of mass spectrometry and structural analysis confirmed that 336mdd was formed as the target compound.
References:
US2022/119325,2022,A1 Location in patent:Paragraph 0100-0121
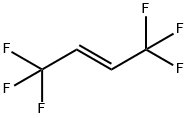
66711-86-2
28 suppliers
inquiry

384-54-3
18 suppliers
$135.00/5G
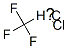
28760-99-8
1 suppliers
inquiry

384-54-3
18 suppliers
$135.00/5G