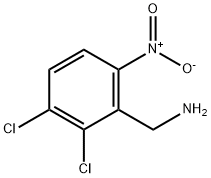
2,3-DICHLORO-6-NITROBENZYLAMINE synthesis
- Product Name:2,3-DICHLORO-6-NITROBENZYLAMINE
- CAS Number:70380-49-3
- Molecular formula:C7H6Cl2N2O2
- Molecular Weight:221.04
136918-14-4
0 suppliers
inquiry

192124-88-2
12 suppliers
inquiry

70380-49-3
41 suppliers
$65.00/100mg
Yield:-
Steps:
Preparation of 2,3-Dichloro-6-nitrobenzylamine
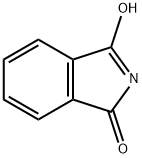
Preparation of 2,3-Dichloro-6-nitrobenzylamine Potassium carbonate and 2 equivalents of phthalimide are ground together and heated to reflux with 2 equivalents of 1,2-dichloro-3-chloromethyl-4-nitrobenzene for three hours. Upon cooling, the substituted benzylphthalimide crystallizes and is filtered in 60-80% yield. 2,3-Dichloro-6-nitrobenzylamine is obtained in a 75-95% yield with acid hydrolysis using hydrochloric acid from the substituted benzylphthalimide.
References:
Roberts Laboratories Inc. US6194420, 2001, B1