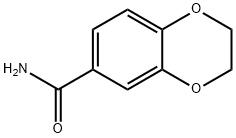
2,3-dihydro-1,4-benzodioxine -6-carboxamide synthesis
- Product Name:2,3-dihydro-1,4-benzodioxine -6-carboxamide
- CAS Number:299169-62-3
- Molecular formula:C9H9NO3
- Molecular Weight:179.17
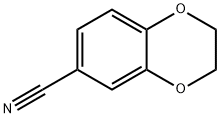
19102-07-9
64 suppliers
inquiry

299169-62-3
29 suppliers
$60.00/100mg
Yield:299169-62-3 74%
Reaction Conditions:
Stage #1: 2,3-dihydrobenzo[b][1,4]dioxine-6-carbonitrilewith sulfuric acid;trifluoroacetic acid for 5 h;Reflux;
Stage #2: with waterCooling with ice;
Steps:
Transformations of benzonitriles 7c, 7d, 8, and 10 in CF3COOH-H2SO4 (general procedure).
General procedure: A solution of 3.5 mmol of 7c, 7d, 8, or 10 in a mixture of 1 mL of concentrated sulfuric acid and 4 mL of trifluoroacetic acid was refluxed for 5 h. The mixture was poured into a mixture of 35 mL of water and 35 g of ice, and the precipitate was filtered off, washed with water, and dried in air. If necessary, the product was recrystallized from an appropriate solvent. Compound 11 was described previously [17]. The yields of 13b and 13c are given in Table 1.
References:
Mochalov;Fedotov;Trofimova;Zefirov [Russian Journal of Organic Chemistry,2018,vol. 54,# 3,p. 403 - 413][Zh. Org. Khim.,2018,vol. 54,# 3,p. 399 - 408,10]
29668-44-8
270 suppliers
$6.00/1g

299169-62-3
29 suppliers
$60.00/100mg

52287-51-1
209 suppliers
$6.00/1g

299169-62-3
29 suppliers
$60.00/100mg