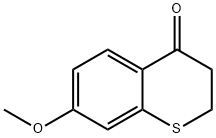
2,3-DIHYDRO-7-METHOXY-4H-1-BENZOTHIOPYRAN-4-ONE synthesis
- Product Name:2,3-DIHYDRO-7-METHOXY-4H-1-BENZOTHIOPYRAN-4-ONE
- CAS Number:13851-03-1
- Molecular formula:C10H10O2S
- Molecular Weight:194.25
![3-[(3-Methoxy)phenylthio] propionic acid](/CAS/GIF/13735-06-3.gif)
13735-06-3
1 suppliers
inquiry

13851-03-1
8 suppliers
inquiry
Yield:13851-03-1 71%
Reaction Conditions:
Stage #1: 3-((3-methoxyphenyl)thio)propanoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane; for 0.5 h;
Stage #2: with aluminum (III) chloride in dichloromethane; for 0.5 h;
Steps:
C 7-Methoxythiochroman-4-one (ld-2):
To a stirred solution of 3-((3-methoxyphenyl)thio)propanoic acid Id-I (12 g, 58.9 mmol) in DCM (120 ml) were added oxalylchloride (8 ml, 55 mmol) and a catalytic amount of DMF (0.5 ml). The reaction mixture was stirred for 30 mm and concentrated to dryness. The residue was dissolved in DCM (30 ml) and was added dropwise to a suspension of aluminum chloride (7.5 g, 56.5 mmol) in DCM (300 ml). The reaction mixture was stirred for 30 mm upon which TLC analysis indicated formation of a less polar product. The reaction mixture was poured into ice cold water and extracted with DCM (2 x 500 ml). The combined organic layers were washed with brine (200 ml), dried over sodium sulfate and concentrated down. The crude product was purified by column chromatography (silica gel 100-200 mesh) using 0-15% EtOAc in petroleum ether to give the title compound Id-2 as an off-white solid (8.1 g, 71% yield). LCMS [M+H] 195.
References:
WO2019/119141,2019,A1 Location in patent:Paragraph 00221; 00236
15570-12-4
235 suppliers
$17.00/1g

13851-03-1
8 suppliers
inquiry