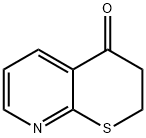
2,3-Dihydropyrano[2,3-b]pyridin-4(4H)-one synthesis
- Product Name:2,3-Dihydropyrano[2,3-b]pyridin-4(4H)-one
- CAS Number:286472-03-5
- Molecular formula:C8H7NOS
- Molecular Weight:165.21
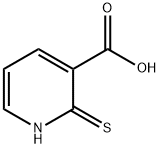
![2-[(2-CARBOXYETHYL)SULFANYL]NICOTINIC ACID](/StructureFile/ChemBookStructure5/GIF/CB6318189.gif)
286472-02-4
16 suppliers
$228.00/1g
![2,3-Dihydropyrano[2,3-b]pyridin-4(4H)-one](/CAS/GIF/286472-03-5.gif)
286472-03-5
10 suppliers
inquiry
Yield:286472-03-5 27%
Reaction Conditions:
with sodium acetate;acetic anhydride at 160; for 2 h;Heating / reflux;
Steps:
21.1
Example 21 Preparation of (5R, 6Z)-7-oxo-6- (4H-thieno [2', 3' : 4, 51thiopyranor2, 3-blpvridin-2- ylmethylene)-4-thia-1-azabicyclo [3. 2. Olhept-2-ene-2-carboxylic acid (Sodium salt) Step 1: 2, 3 dihvdro-4H-thiopyranor2, 3-blpyridin-4-one : A solution of 14 g. (61.6 mmol) 3- (3-Carboxy-2-pyridylthio) propionic Acid [prepared as described in lit. : J. Heterocyclic Chem.. 37. 379 (2000)] and 15 g. (185 mmol, 3 eqs) of anhydrous sodium acetate, in 200 mL. of acetic anhydride was refluxed (160° C) under stirring, N2 atm, dry conditions, for 2 hours. Cooled, diluted with 300 mL of water, basified with 30% ammonium hydroxide solution to pH 8-9, extracted with 3x200 mL chloroform. Combined organics washed with 2x60 mL Sodium bicarbonate (satn. sol), water, dried, evaporated, gave 2.8g. (27%) of the title compound, reddish solid, m. p. 66-8° C, (M+H) +=166. 2.
References:
WO2003/93280,2003,A1 Location in patent:Page/Page column 136
38521-46-9
277 suppliers
$6.00/5g
![2,3-Dihydropyrano[2,3-b]pyridin-4(4H)-one](/CAS/GIF/286472-03-5.gif)
286472-03-5
10 suppliers
inquiry